Bromination of alkenes with Br2 to give dibromides
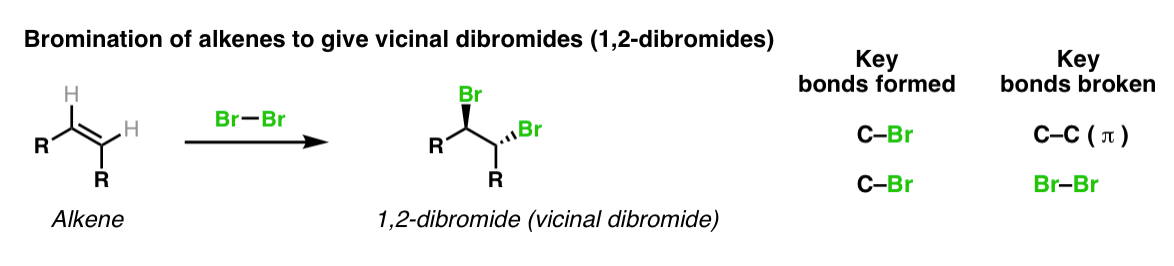
Description: Treatment of alkenes with bromine (Br2) gives vicinal dibromides (1,2-dibromides).
Notes: The bromines add to opposite faces of the double bond (“anti addition”). Sometimes the solvent is mentioned in this reaction – a common solvent is carbon tetrachloride (CCl4). CCl4 actually has no effect on the reaction, it’s just to distinguish this from the reaction where the solvent is H2O, in which case a bromohydrin is formed (see also).
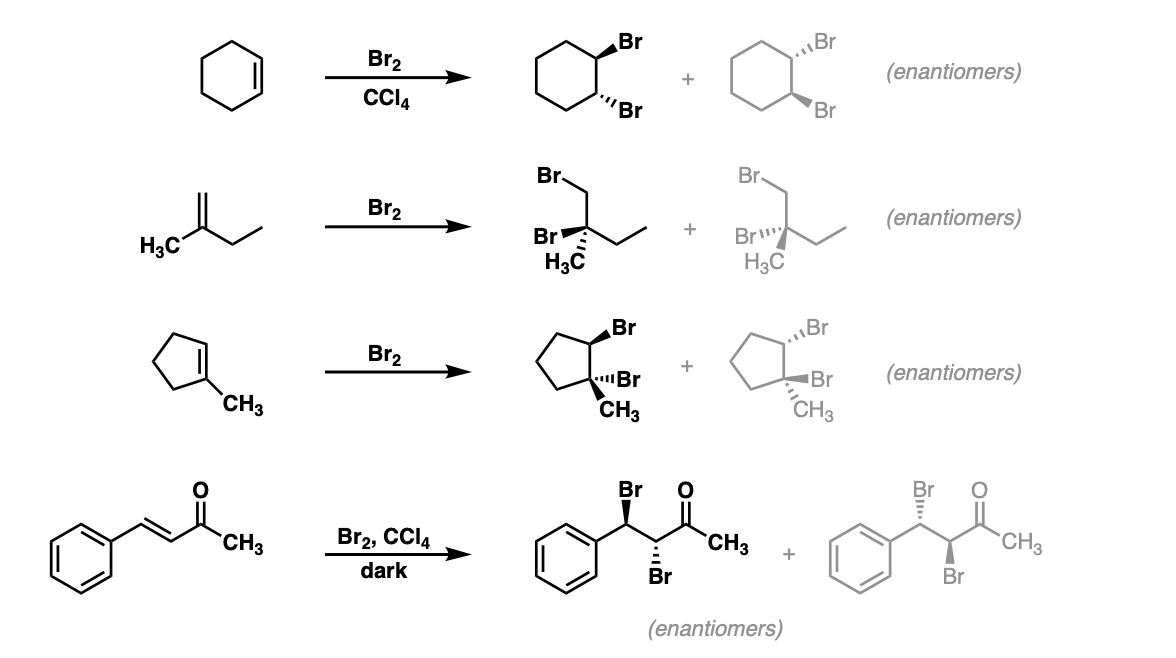
Notes: Again note that in the first example CCl4 is merely the solvent and in these cases has no effect on the reaction (unlike when water or alcohols are the solvent – see bromohydrin formation page). Sometimes “dark” is mentioned to distinguish this reaction from cases where Br2 can promote bromination through a radical pathway.
Mechanism:
Attack of the alkene on bromine (Step 1, arrows A and B) gives the bromonium ion, which is attacked at the backside by bromide ion to give the trans-dibromo product. Note that the bromines are delivered to opposite sides of the alkene (“anti” addition).
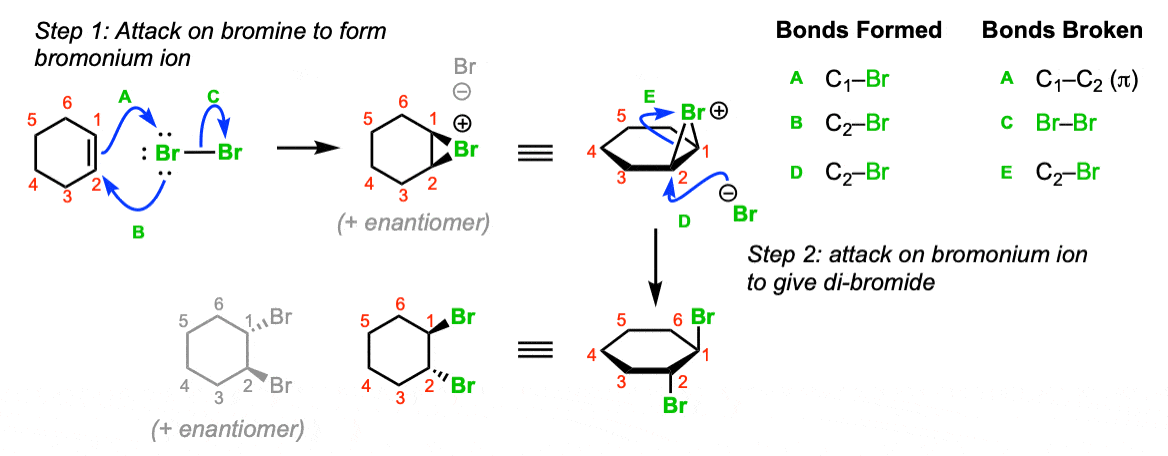
Notes: Note that a 1:1 mixture of enantiomers will be formed in this reaction. The enantiomer formed will depend on which face of the alkene the bromine adds to.
Additional examples:
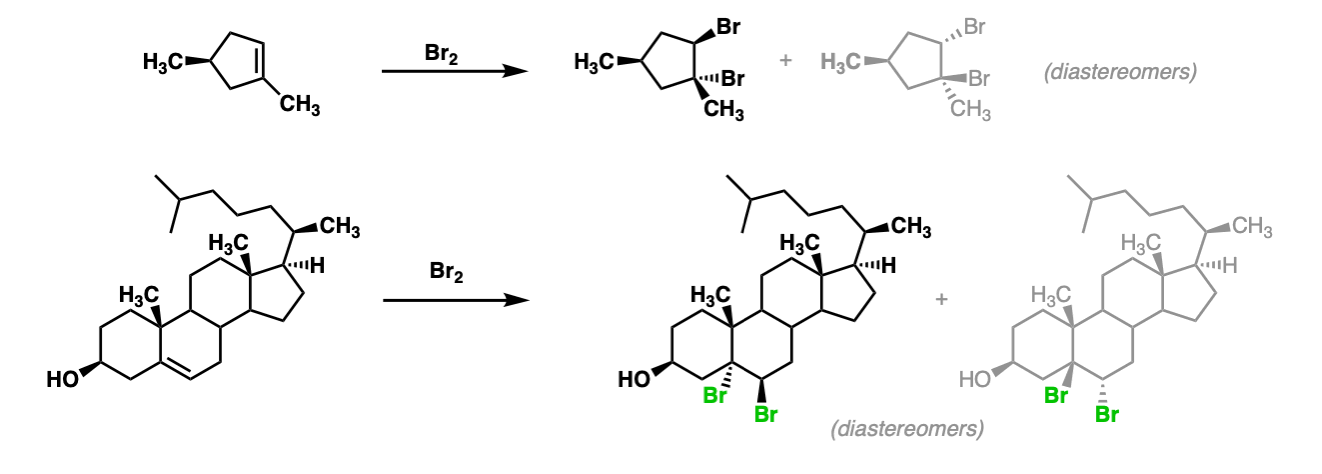
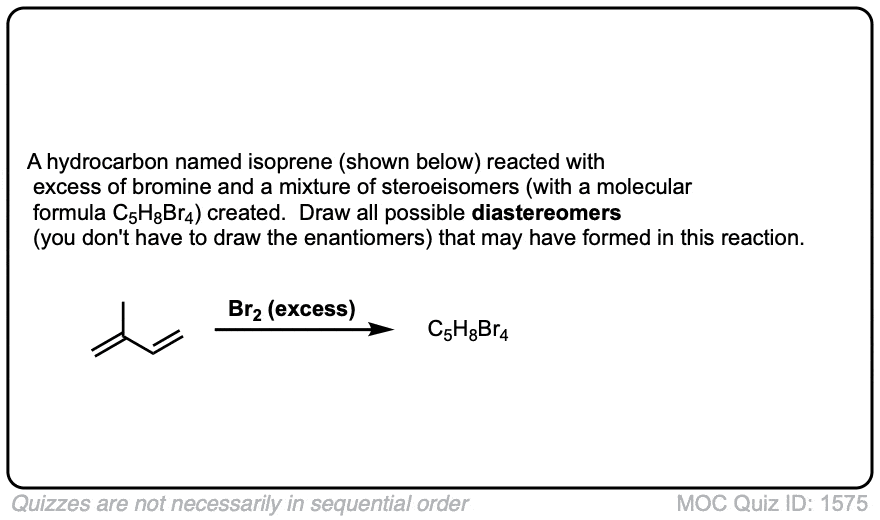
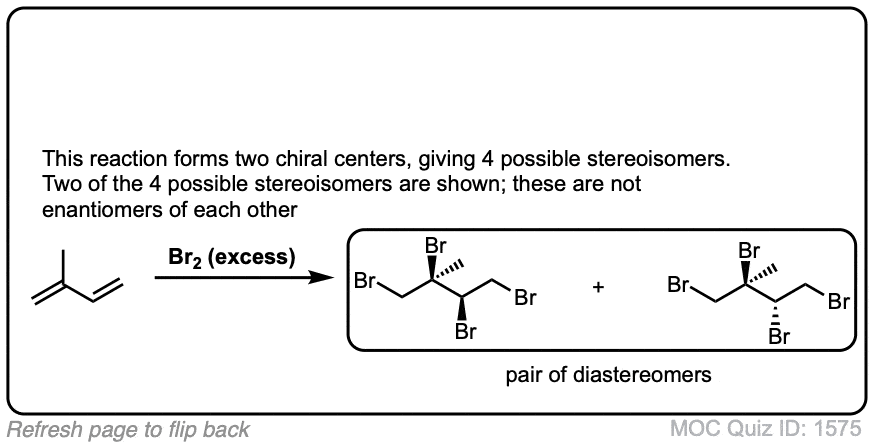
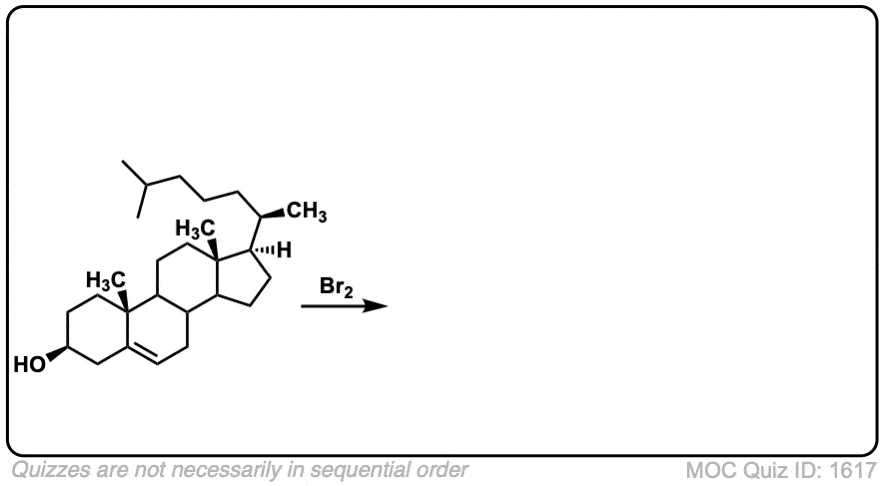
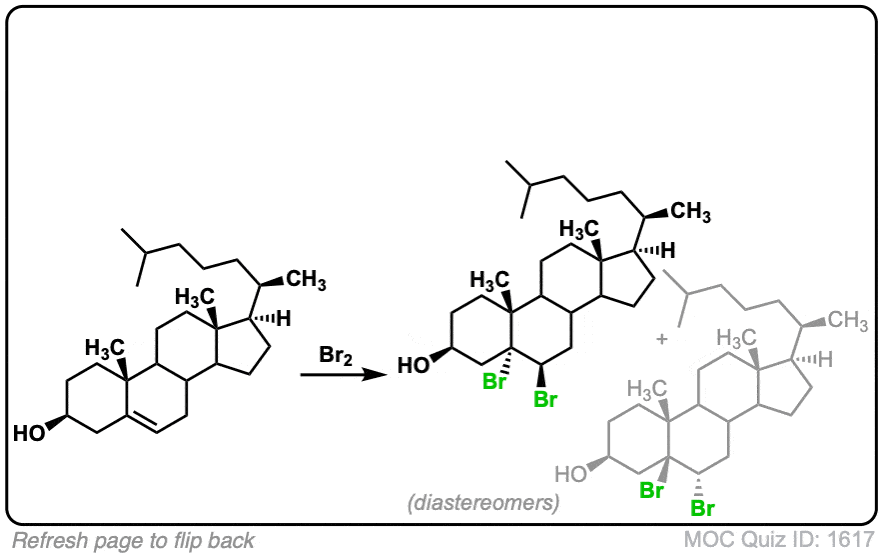
These examples show what happens when a chiral center is already present on the molecule; a mixture of diastereomers is obtained.
The bottom example looks complicated, but actually isn’t – we’re still just breaking C-C, and forming two C-Br bonds, just as with any other alkene. Try not to get freaked out by all the additional atoms in the molecule! (There are chiral centers in the starting material, so again we’re forming a mixture of diastereomers).
Quiz Yourself!
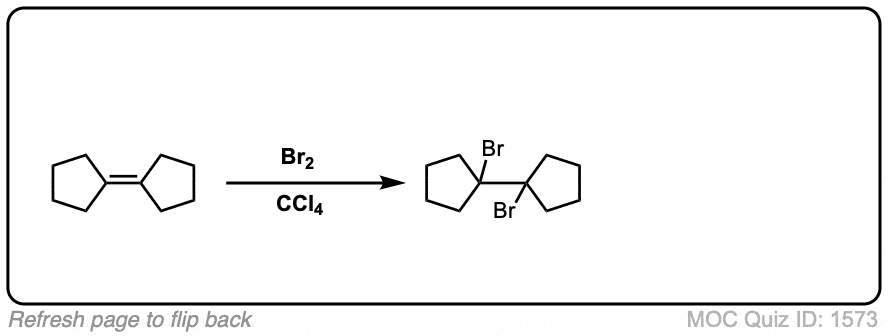
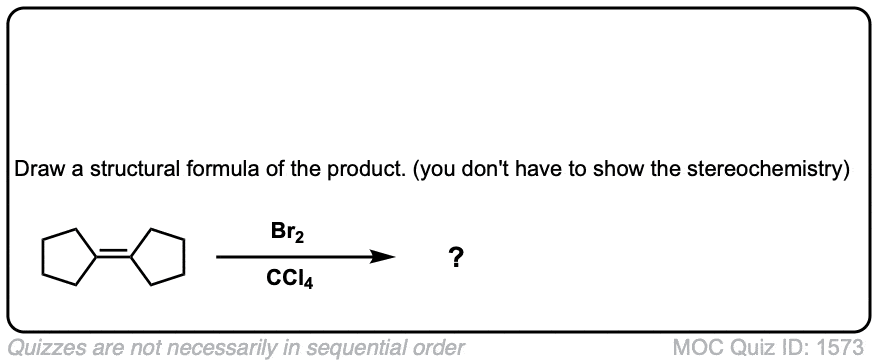 Click to Flip
Click to Flip
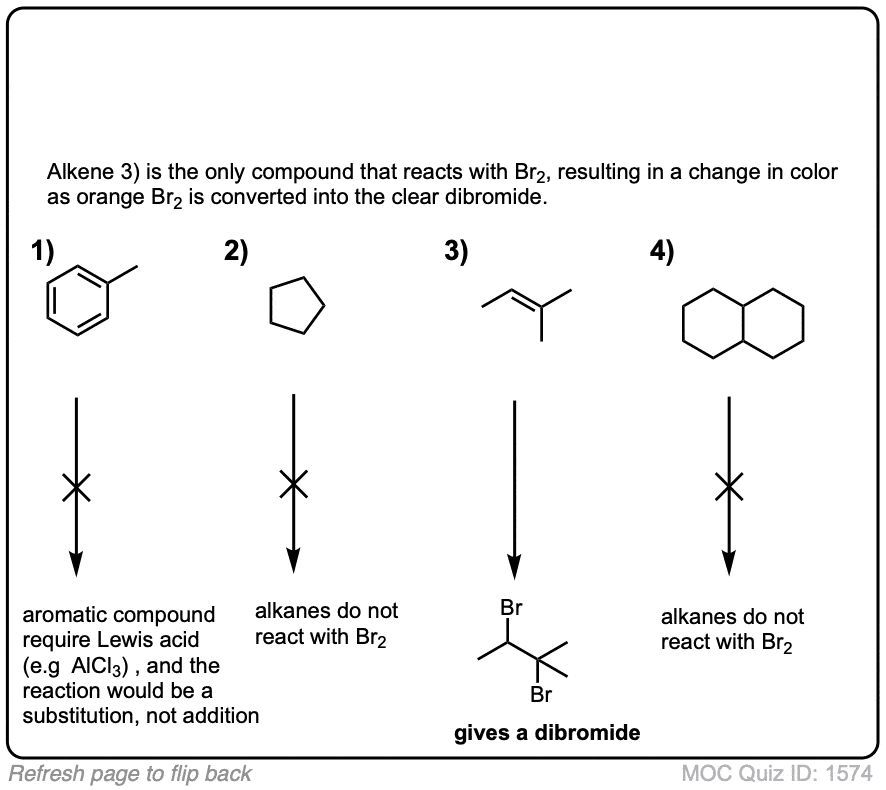
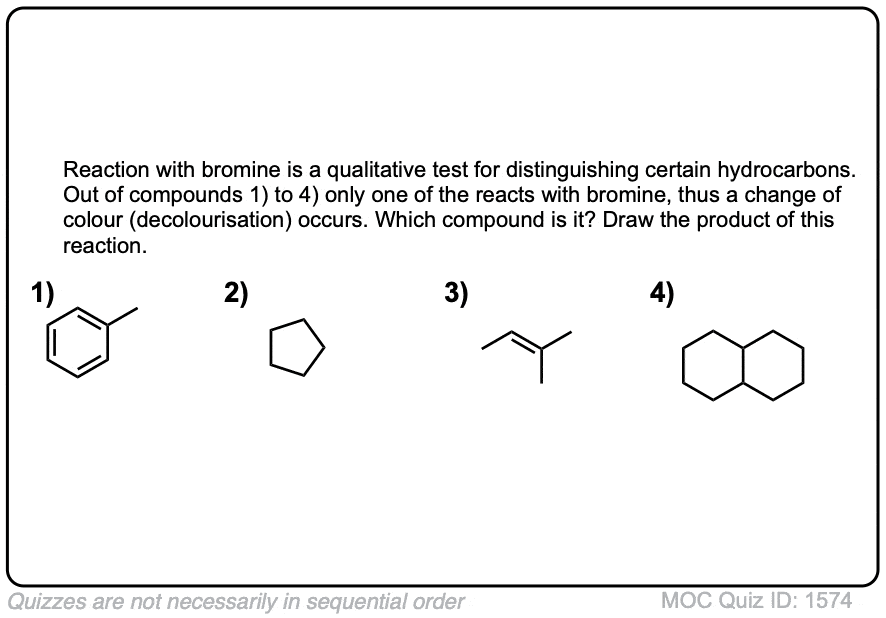 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
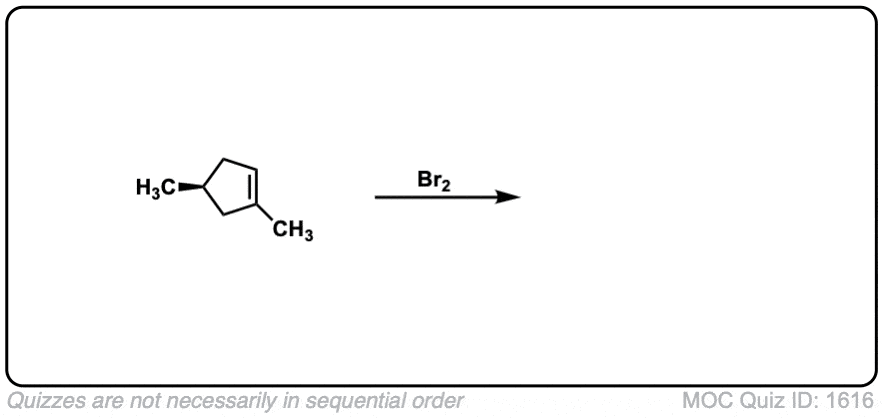
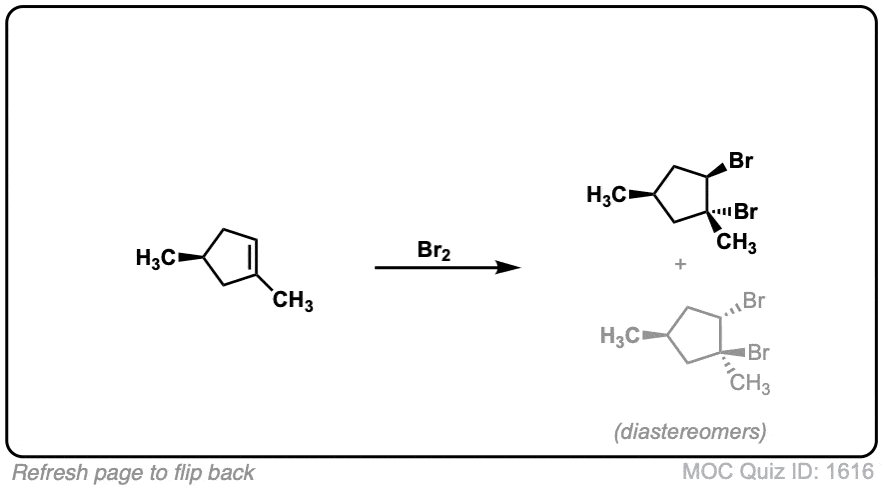
Exam-Type Examples
 Click to Flip
Click to Flip
 Click to Flip
Click to Flip
(Advanced) References and Further Reading:
- First example
The Halogenation of Ethylenes
Irving Roberts and George E. Kimball
Journal of the American Chemical Society 1937 59 (5), 947-948
DOI:10.1021/ja01284a507
One of the earliest descriptions in the literature of a three-membered bromonium ion, accounting for the anti stereochemistry of this reaction. - Mechanistic studies
The question of reversible formation of bromonium ions during the course of electrophilic bromination of olefins. 2. The crystal and molecular structure of the bromonium ion of adamantylideneadamantane
Slebocka-Tilk, R. G. Ball, and R. Stan Brown
Journal of the American Chemical Society 1985 107 (15), 4504-4508
DOI: 10.1021/ja00301a021
This paper describes the crystal structure of an isolated, stable bromonium ion. This is significant because it proves the intermediacy of these three-membered cyclic bromonium ions in the electrophilic addition of bromine to alkenes. - Reference To An Experimental Procedure
Rufine Akué-Gédu and Benoît Rigo
Org. Synth. 2005, 82, 179
DOI: 10.15227/orgsyn.082.0179
Real Life Examples:
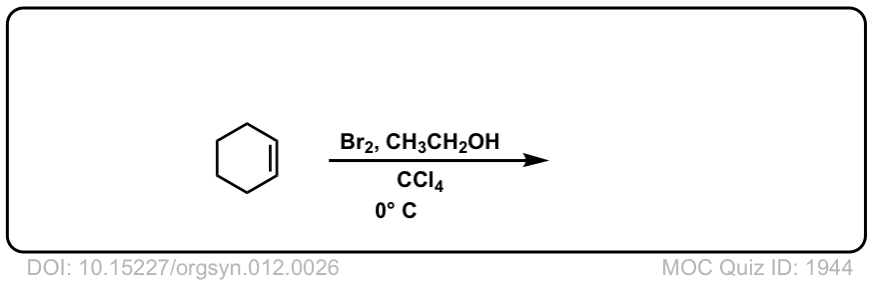
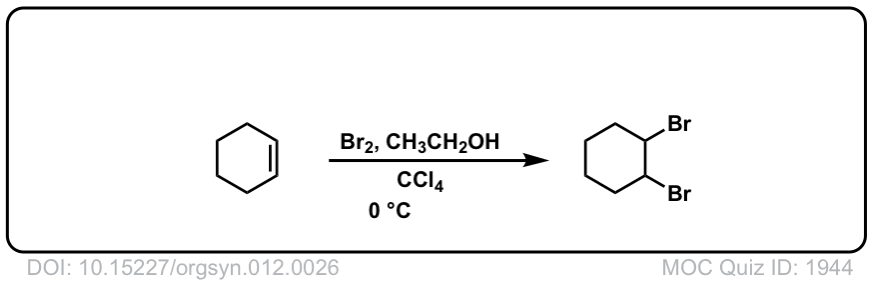
Org. Synth. 1932, 12, 26
DOI Link: 10.15227/orgsyn.012.0026
 Click to Flip
Click to Flip
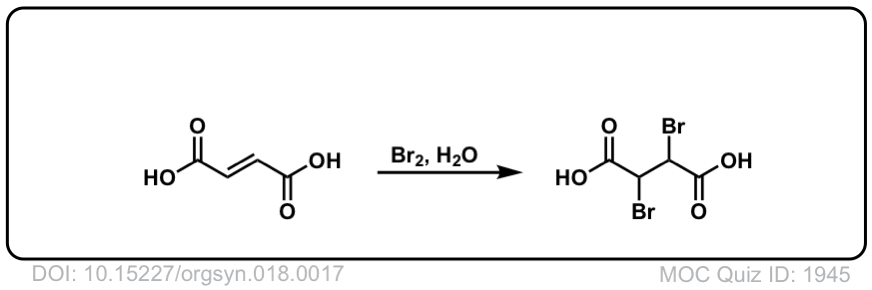
Org. Synth. 1938, 18, 17
DOI Link: 10.15227/orgsyn.018.0017
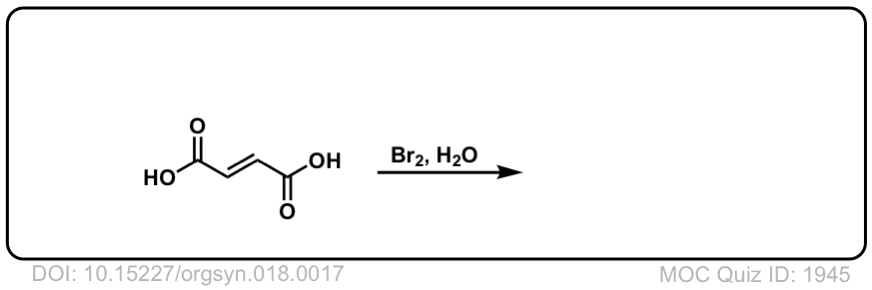 Click to Flip
Click to Flip
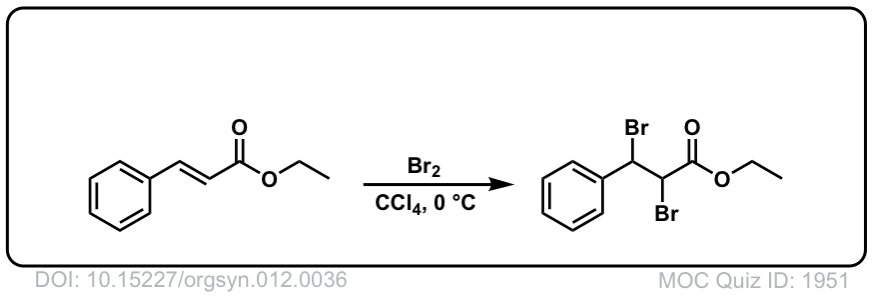
Org. Synth. 1932, 12, 36
DOI Link: 10.15227/orgsyn.012.0036
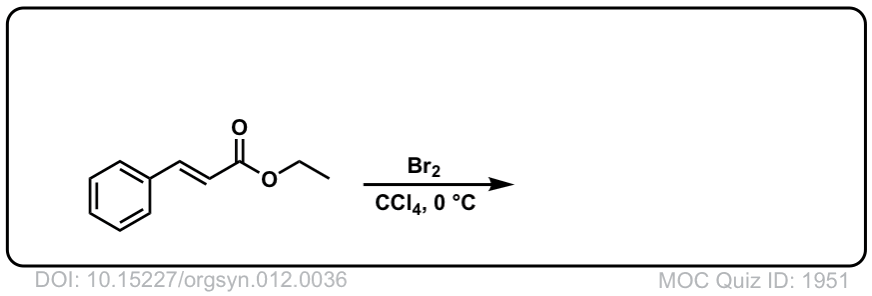 Click to Flip
Click to Flip
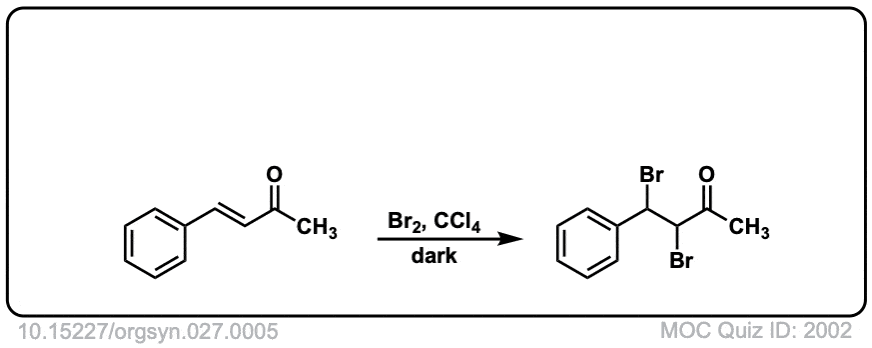
Org. Synth. 1947, 27, 5
DOI Link: 10.15227/orgsyn.027.0005
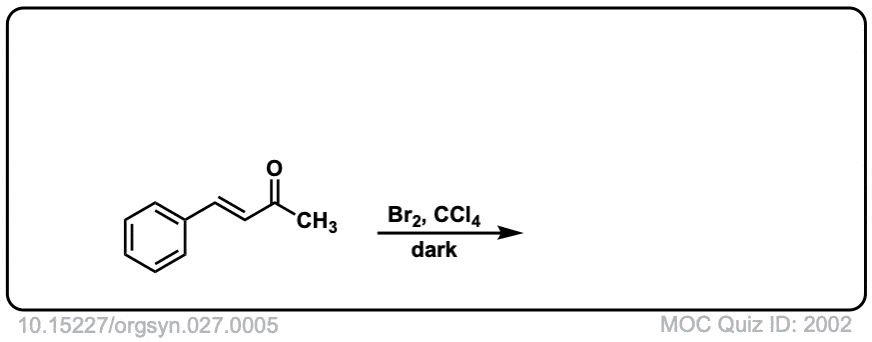 Click to Flip
Click to Flip
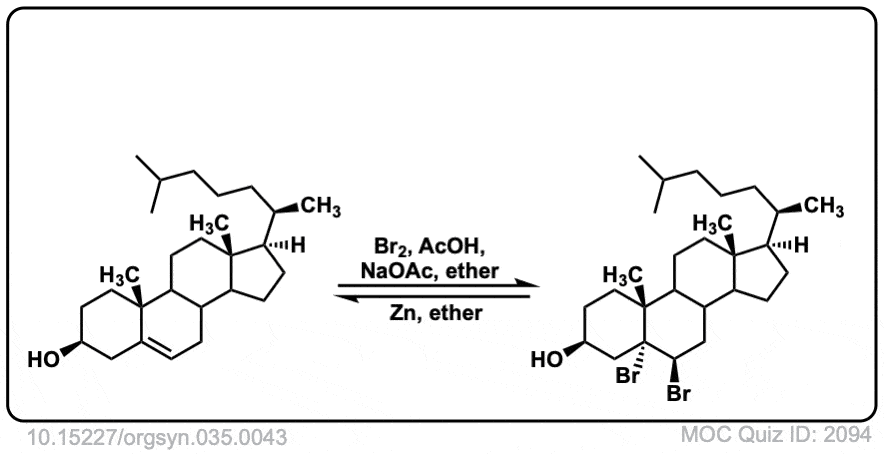
Org. Synth. 1955, 35, 43
DOI Link: 10.15227/orgsyn.035.0043
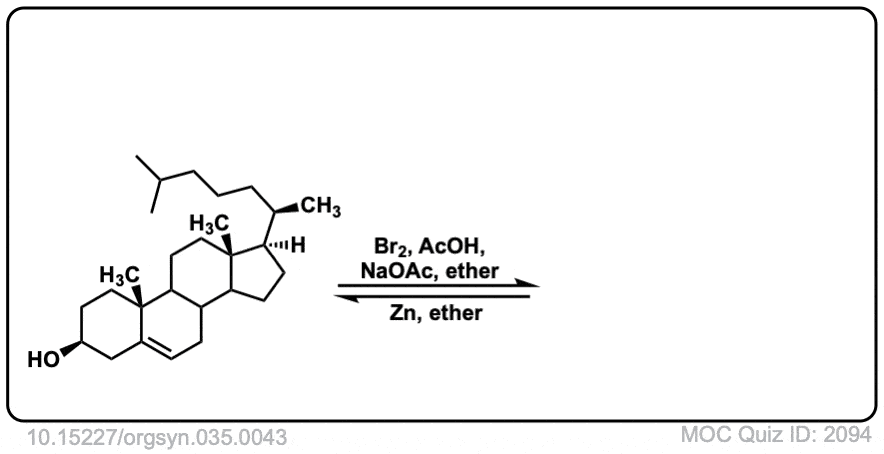 Click to Flip
Click to Flip
Org. Synth. 1957, 37, 77
DOI Link: 10.15227/orgsyn.037.0077
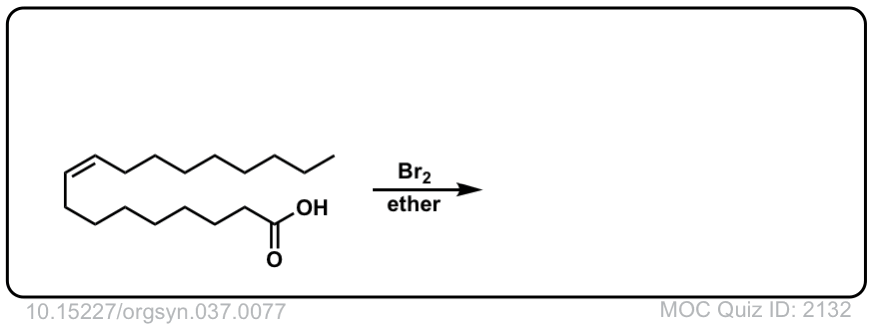 Click to Flip
Click to Flip
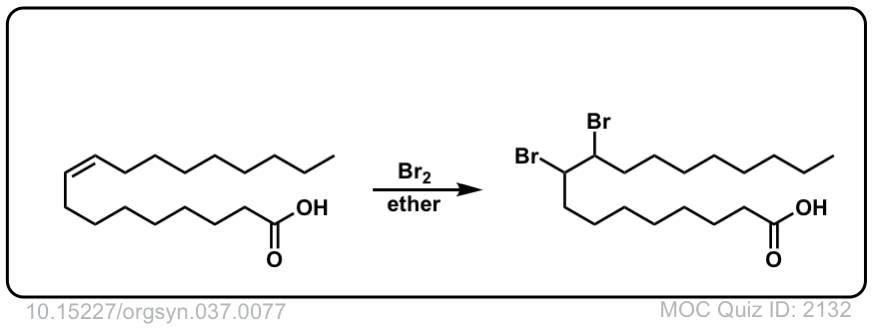
Org. Synth. 1952, 32, 104
DOI Link: 10.15227/orgsyn.032.0104
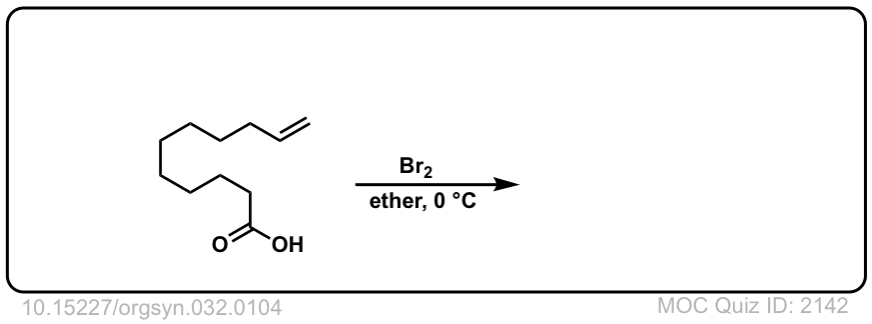 Click to Flip
Click to Flip
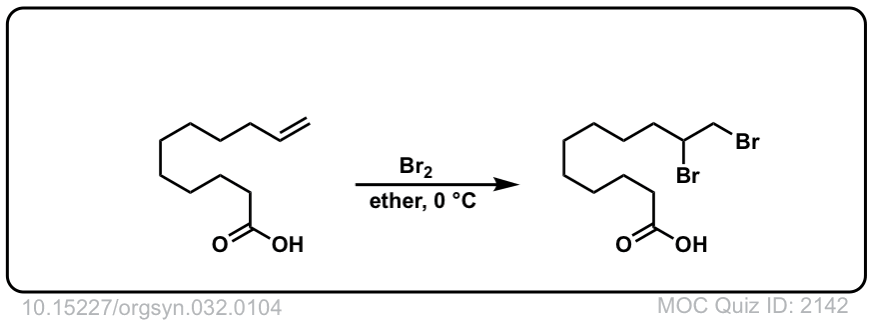
Org. Synth. 1952, 32, 104
DOI Link: 10.15227/orgsyn.032.0104
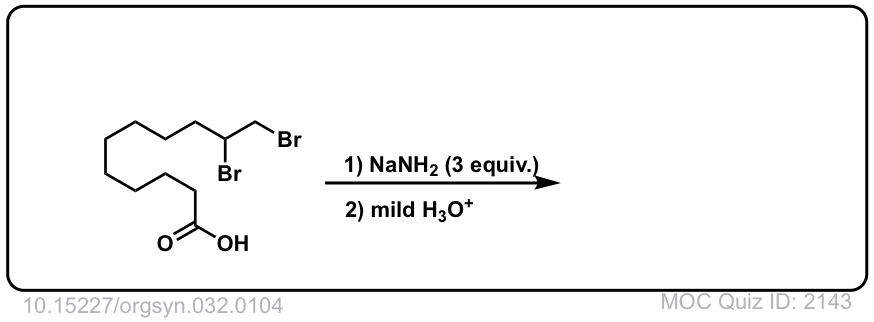 Click to Flip
Click to Flip
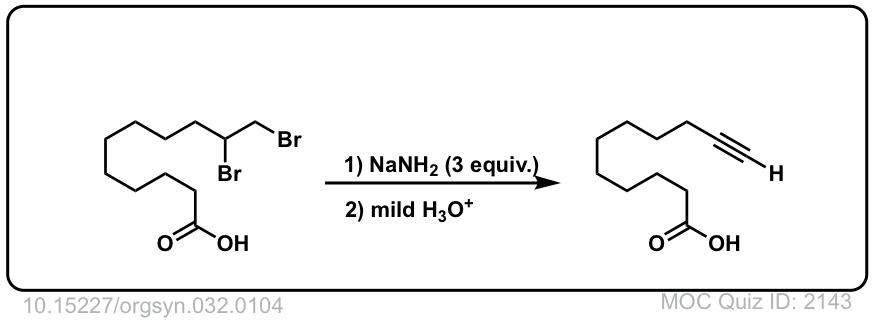
Org. Synth. 1952, 32, 104
DOI Link: 10.15227/orgsyn.032.0104
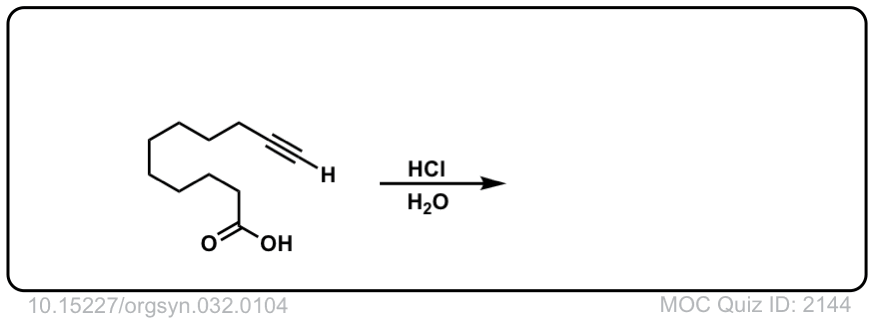 Click to Flip
Click to Flip
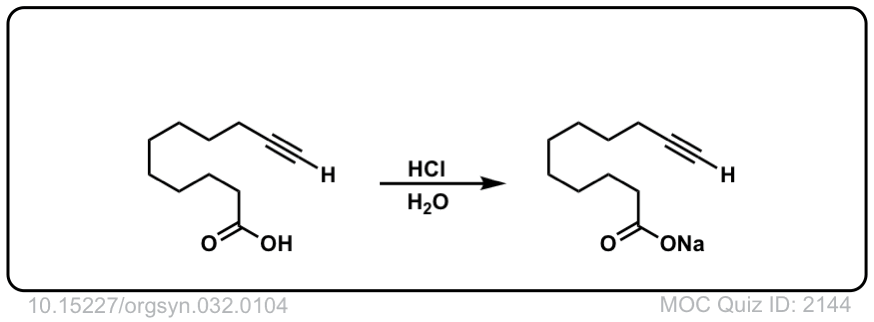
The quiz 2144, why does HCl, H2O give deprotonation of the carboxylic end of the molecule? Why wouldn’t we instead see something to the effect of:
1) Protonation of the carbonyl oxygen
2) Followed by nucleophilic attack by free electron pair on H2O oxygen (and pi-electrons to positive oxygen on carbonyl)
3) Deprotonation of the -OH2 group to yield another -OH group.
Final product would be 3 -OH on the terminal carbon (carbonyl carbon). Although I don’t think this reaction would occur it does seem more likely than deprotonation of carboxylic proton in a very acidic environment.
Thanks for pointing this out. The product and starting materials should be reversed.
Not only that it has nothing to do with bromination. These quizzes shouldn’t really even be on this page. Moved.
reason for reactivity order of halogens towards alkenes f2>Cl2>br2>I2.why vicinal diiodides are unstable at room temperature?
The I-I bond is very weak (about 40 kcal/mol) and easily broken, even by visible light.
What would be the products in case of reaction of cis and trans cyclooctene with Br2/CCl4?
Since halogenation is stereospecific, they would be stereoisomers of each other,
any real world examples of bromination in human physiology?
No, electrophilic bromine tends to be pretty rare in humans.
hi, i found out that bromine water has H2O molecules, since there is H2O (polar solven), it will act as an electrophile which “interferes” the mechanism and it will acts as an electrophile to attack the bromide ion. The product formed will be bromohydrin.This process is known as halohydrin. But here you said that, bromine will react with the alkene and form 1,2-dibromides, i am confused about these two reactions. Can you explain? :<
It is all about solvent choice.
Bromine water is Br2 / H2O, and when one brominates an alkene with bromine water, you are correct in that you obtain a halohydrin.
To obtain a dibromide product, you have to use Br2 in an inert solvent such as CCl4 or CH2Cl2.
Hi dude!
Just loved your notes a lot!
I have a small doubt. If a hydrocarbon reacts with Br2 in the dark, and has Degree of unsaturation as 1, what isit? Alkene or alkane?
You can figure this out!
Thank you so much, i really needed this info.
How does conjugation effect the electrophilic addition of Br2 to a molecule?
Great question. See this post: https://www.masterorganicchemistry.com/2017/04/11/more-on-12-and-14-additions-to-dienes/
What if alkene is treated with only bromine at high temperature ? (NO CCl4)
Running this reaction neat (i.e. without solvent) is generally not recommended, as it will generate heat. Solvent helps to keep the temperature uniform and the reaction rate under control. Bromination is a fast reaction even at 0°C so heating is not necessary. Heating it is generally overkill. On anything but the simplest molecules the result will likely be a mess.
hi!
for the compound (C(CH3)3)2C=CH2
on the addition of br2 will form first the cyclic bromonium ion
now since the other carbon is sterically hindered how will br- attack that carbon.
In that specific example you couldn’t tell the difference because it does not lead to isomers. But I would still expect Br- to attack the more substituted carbon.
what is the role of ccl4 in anti addition when halogen is added to an alkene??
It’s just the solvent. It doesn’t participate.
This is fine… but will the reaction take the same course even in the presence of chloroform instead of carbon tetra chloride..? Does chloroform involve in any way in the reaction?
No, it’s merely the solvent. Has no effect.
I take issue with your incomplete curly arrows for the formation of the cyclic bromonium ion. You’ve missed a third curly arrow going from a bromine lone pair back onto one of the alkene’s carbon atoms. The MO theory model of Br2 bromination of an alkene shows that there is concomitant donation of the alkene HOMO into the bromine sigma-antibond, and a sort of “backbonding” of the bromine pi-antibond HOMO (filled!) into the pi-antibond LUMO of the alkene.
You should correct the above incomplete mechanism, especially seeing as a lot of students use this website as a resource!
if in a reaction ,only br2 is mentioned than will the product would be cis or trans???
i’m so confused that when and where ,in how many rxns in organic there is syn addn and elimination and where its trans??
For alkenes, “concerted” reactions tend to be syn. See: https://www.masterorganicchemistry.com/2013/04/02/alkene-addition-pattern-3-the-concerted-pathway/
Reactions with “anti” stereochemistry tend to go through the 3-membered ring pathway: https://www.masterorganicchemistry.com/2013/03/20/alkene-addition-pattern-2-the-three-membered-ring-pathway/
After the cyclic intermediate, why the other Bromine ion attacks at the point why has more +I effect? Shouldn’t it attack at the other end?
Can you brominate pyrrole with Br2 by just mixing them and letting them sit for awhile?
Yes, pyrrole is an electron-rich heteroaromatic that will react with Br2 in the absence of a catalyst. It will attach at C-3
is the reaction of phenol and bromide the same as cyclohexene are they both addition reaction?
Phenol is aromatic and does not undergo addition reactions. Instead it will undergo electrophilic aromatic substitution. https://www.masterorganicchemistry.com/2017/07/11/electrophilic-aromatic-substitution-introduction/
Which are the solvents that can dissolve both bromine and NaBr to give solutions containing both Br2 and NaBr?
Why would you use NaBr and not something like Bu4N+ Br- ?
What would happen if bormination were to occur with a diene? There is no other intermediate besides the bromonium ion.
Specifically how does the rearrangement occur?
Is the first Br considered slightly positive just before it is attacked by the electron dense double bond? I’m just trying to understand why the electrons from the double bond are attracted to the Br. Thanks!
No, the Br-Br bond is perfectly covalent. But what does happen is that one of the Br atoms (at random) is attacked by the pi bond, and something called a “pi complex intermediate” is formed. This breaks down (losing Br-) to give the 3-membered ring.
Great post here, one question- could you discuss what would happen when 1,2 dimethylcyclohexene reacts with Br2 (1 mole)/light? The answer key shows a double bond shift and only one Br attaching. Everywhere I’ve read, in an alkene reaction both Br’s attache to the ring in trans.. Could you show the mechanism involved?
I believe only 1 Br attaches because it is a radical reaction, hence the “light”. Being radical means only 1 attaches during propagation
Yeah I just looked this up because I was confused too. When you have light plus Br2 plus an alkene you get allylic bromination, which means a bromine radical removes a hydrogen (and one electron from its bond) from a carbon adjacent to the double bond (in allylic position), leaving only one electron on that carbon which reacts with another Br2 to take one of the bromines and one of its electrons to form a halogenated alkene and another bromine radical in the propagation step. If you have an alkene plus light plus HBr, the light forms a bromine radical which forms a bond to the least substituted carbon of the alkene and puts a radical on the most substituted carbon, which then grabs the hydrogen and one electron from another HBr molecule, forming another bromine radical and making it a propagation step.
When you don’t have light but have Br2 and an alkene and a non-nucleophilic solvent the bromines add to both carbons that formerly held the double bond- the first bromine is grabbed by the pi bond and leaves its electrons to form a ring with the alkane (halonium ion), which the other bromine (which has extra electrons and is thus nucleophilic) then attacks from the back, which breaks the ring and forms an alkane with two bromines attached to adjacent carbons. If you add HBr with a non-nucleophilic solvent and without light the same thing happens, but the first atom grabbed by the pi bond is the hydrogen without its electrons. (In this process the hydrogen doesn’t form a ring, just a carbocation, so rearrangement can occur to generate different di-halogenated alkanes.)
So basically radical addition of halogens to a pi bond (that gives the anti-Markovinov product) only happens if you have HBr and light (and peroxides to create the bromine radical), not Br2 and light. If you have Br2 and light, allylic bromination occurs, which adds Br not to the double bond but to the carbon adjacent to the doubly bonded carbons. Or the bromine can just add across the double bond to each of the formerly double bonded carbons, as our dear Ochem master says on his page:
Allylic bromination: https://www.masterorganicchemistry.com/2013/11/25/allylic-bromination/
Free Radical Addition of HBr to Alkenes: https://www.masterorganicchemistry.com/2013/04/12/a-fourth-alkene-addition-pattern-free-radical-addition/
Thanks. That cleared everything.
why does Bromination ALWAYS form an anti-addition product?
I’ve tried to make this as simple as I can.
Unlike Bromohydrin. Why isn’nt there any chlorohydrin?Is that not possible?
Unless I’m very mistaken, only the Br-Br bond is weak enough to be broken in the presence of an alkene.
Cl2 and I2 work as well, among others.
In the reaction of alkenes with Cl2 and water, chlorohydrins are formed.