Iodination of alkenes to give vicinal diiodides (1,2-diiodides)
Description: Treatment of alkenes with iodine (I2) leads to the formation of vicinal diiodides (1,2-diiodides).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
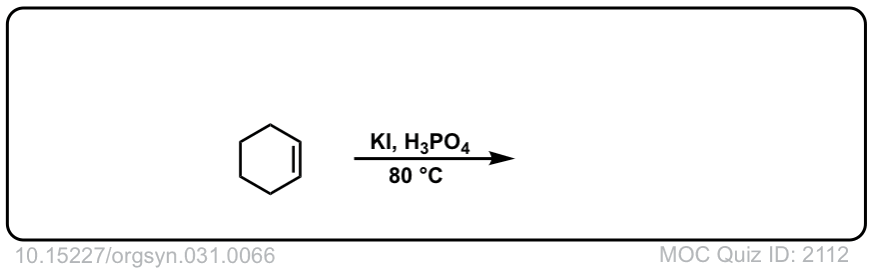
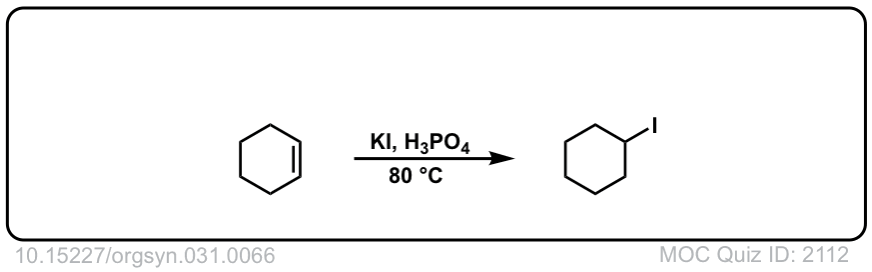
Org. Synth. 1951, 31, 66
DOI Link: 10.15227/orgsyn.031.0066
 Click to Flip
Click to Flip
How is the 5th example a meso compound when there is no internal line of symmetry? one iodine is on a wedge while the other is on a dashed line.
I hate to nitpick little things like this (this resource is amazing I really appreciate it). I noticed the formation of the Iodonium ion in the main mechanism uses lines for the C-I connections, while in other examples a wedge was used
From one nitpicker to another, thank you. Easy thing to fix (done). Anything else, just let me know! James
Since this is anti-additon, in the fourth example, shouldn’t the I’s on the third and fourth Carbon’s be one wedged and one dashed not both wedged and dashed?
If you look closely the central carbon-carbon bond has also rotated. If that rotation had not happened, then the two C-I bonds would be anti.
This is a very common type of exam problem, where the question will start with an alkyne and then do either Lindlar or Na/NH3 to give a cis- or trans- alkene, and then do either a syn- or anti- selective reaction in the second step.
[wp_quiz_pro id="19026"]