Addition Of Alcohols To Alkenes With Acid
Description: Alkenes treated with acid in the presence of an alcohol (usually as solvent) form ethers.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
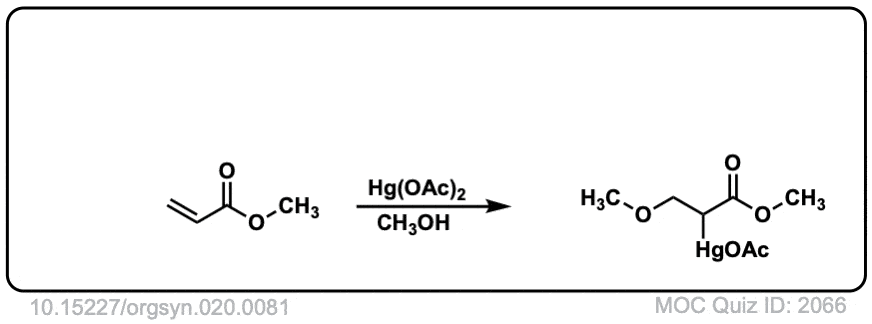
Org. Synth. 1940, 20, 81
DOI Link: 10.15227/orgsyn.020.0081
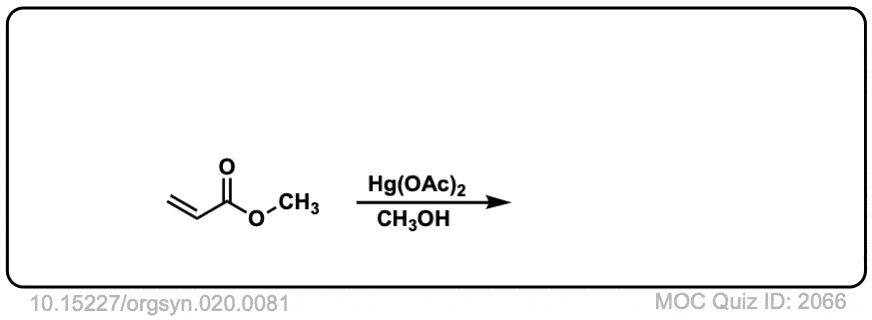 Click to Flip
Click to Flip
so the H from the OH does not bond to anything?
can you explain the mechanism of the intramolecular reaction in example #4??
Sure. First step is that the alkene reacts with H+ to form C-H on the less substituted carbon, leaving behind a tertiary carbocation. This tertiary carbocation is 5 bonds away from the oxygen. 5 membered rings are nice and easy to form. So the oxygen lone pair attacks the carbocation generating a new 5- membered ring which is then deprotonated.
Intramolecular reactions are great exam questions.
During the final deprotonation step, does one have to consider the pKa’s of both the R-OH and the H+ in order to properly write out the mechanism?
I don’t think so. There’s often multiple species in solution that are competent to act as a base on a strong acid like protonated alcohol (pKa -3). I drew HSO4- here to complete the catalytic cycle but honestly it could be a number of different species. That’s why people often just like to write, “B” for generic base.