Formation of carboxylic acids from Grignard reagents and CO2
Description: Grignard reagents will add to carbon dioxide, forming carboxylate salts. After acidic workup, carboxylic acids are formed.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
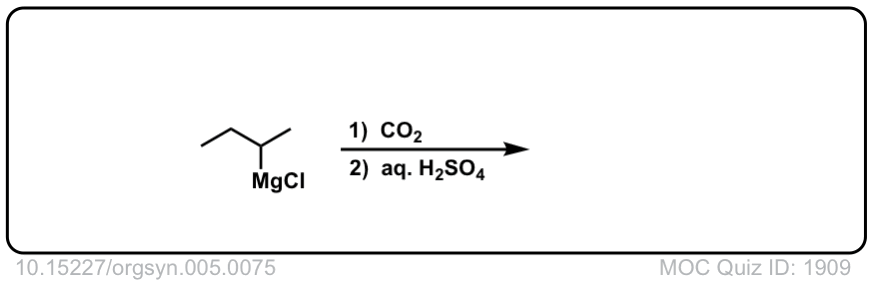
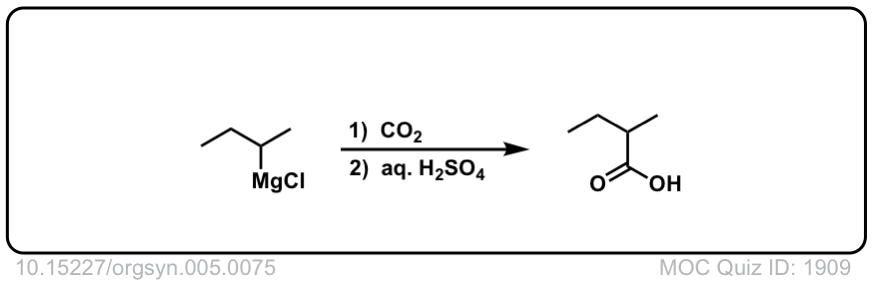
Org. Synth. 1925, 5, 75
DOI Link:10.15227/orgsyn.005.0075
 Click to Flip
Click to Flip
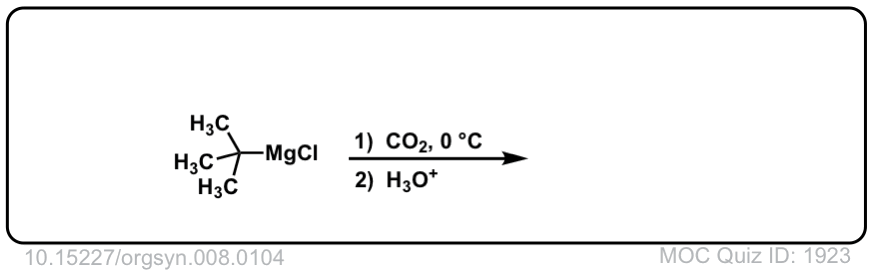
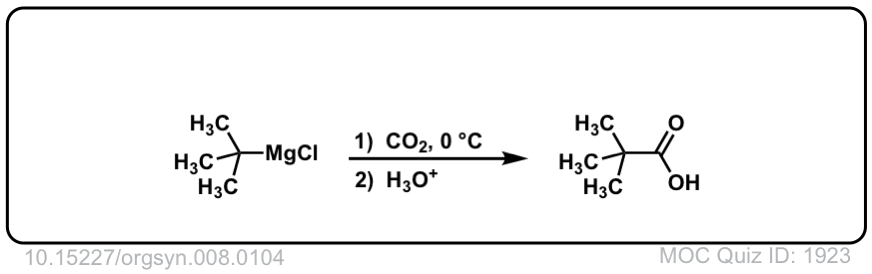
Org. Synth. 1928, 8, 104
DOI Link: 10.15227/orgsyn.008.0104
 Click to Flip
Click to Flip
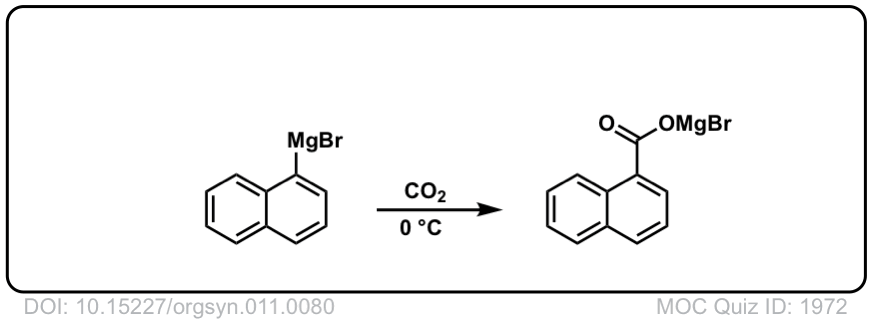
Org. Synth. 1931, 11, 80
DOI Link: 10.15227/orgsyn.011.0080
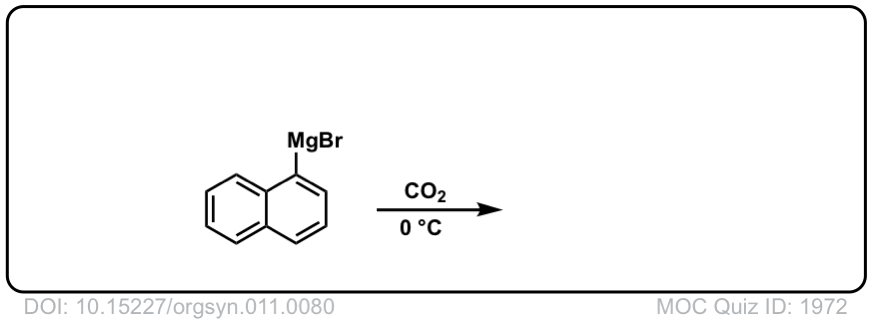 Click to Flip
Click to Flip
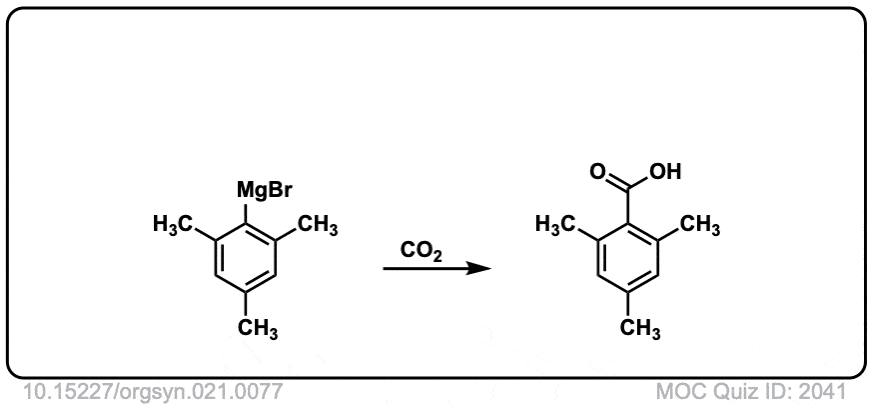
Org. Synth. 1941, 21, 77
DOI Link: 10.15227/orgsyn.021.0077
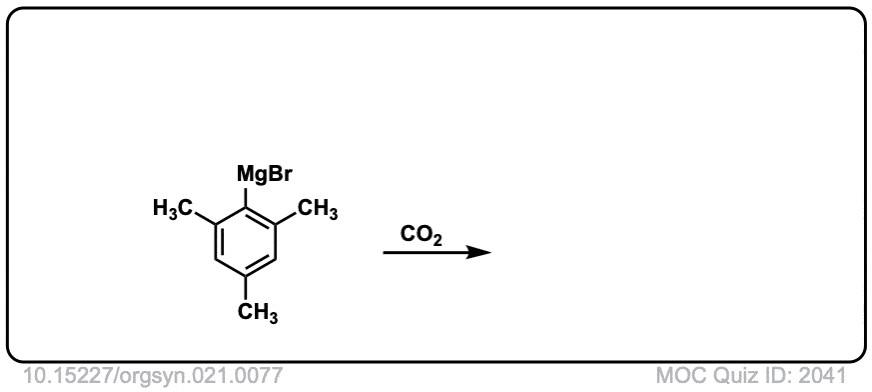 Click to Flip
Click to Flip
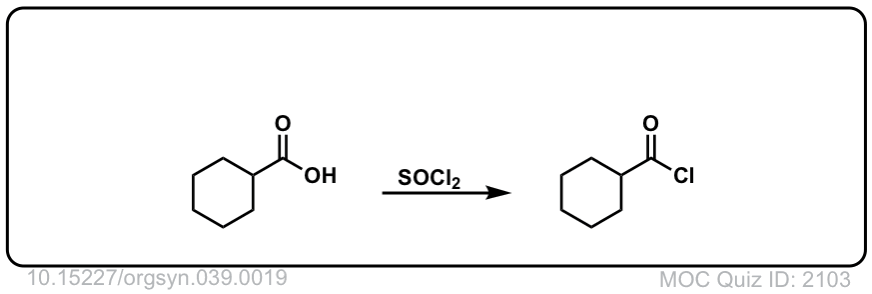
Org. Synth. 1959, 39, 19
DOI Link: 10.15227/orgsyn.039.0019
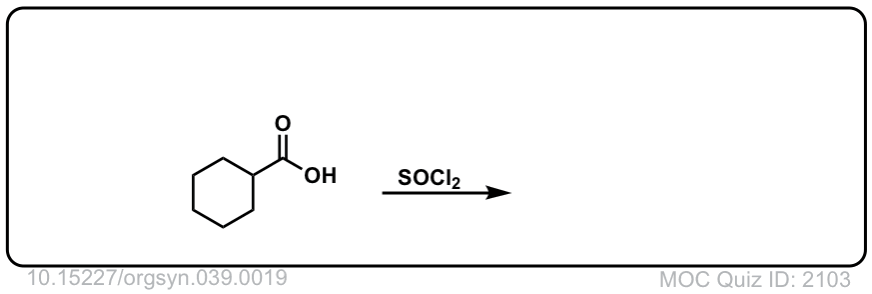 Click to Flip
Click to Flip
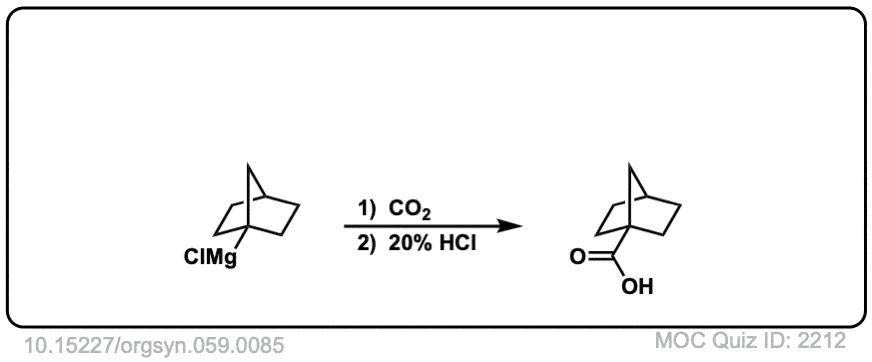
Org. Synth. 1979, 59, 85
DOI Link: 10.15227/orgsyn.059.0085
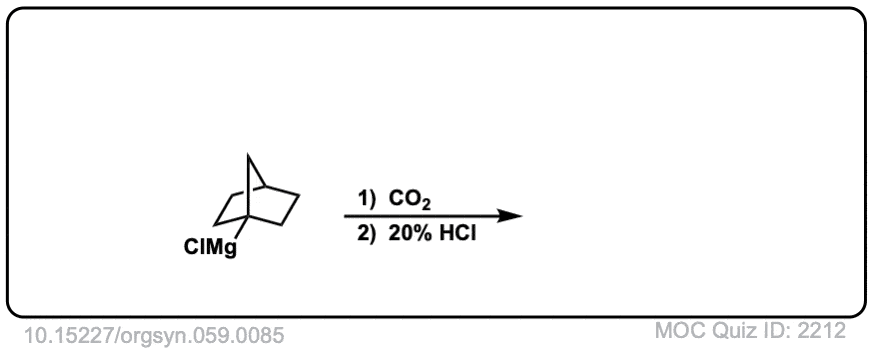 Click to Flip
Click to Flip
help me find the reaction mechanism for the formation of carboxylic acids from carbon (iv) cxide by grignard synthesis
It is not a complicated mechanism. The Grignard adds to CO2 as it would to any other carbonyl, and the resulting carboxylate is then treated with acid to give the neutral carboxylic acid. See: https://www.nature.com/articles/ncomms6933
Wow! This helps me very much!
Thank you so much!
wow! this page is so sexy!