Opening of epoxides with nucleophiles under acidic conditions
Description: When acid is added to epoxides, nucleophiles will add to the more substituted position. The stereochemistry of the addition is trans.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
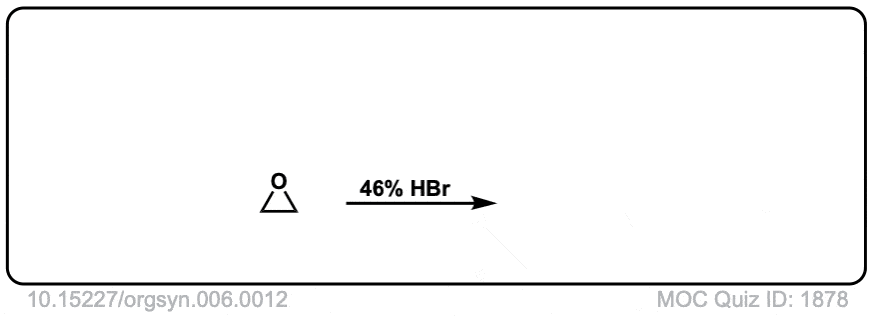
Real-World Example:
Org. Synth. 1926, 6, 12
DOI Link: 10.15227/orgsyn.006.0012
 Click to Flip
Click to Flip
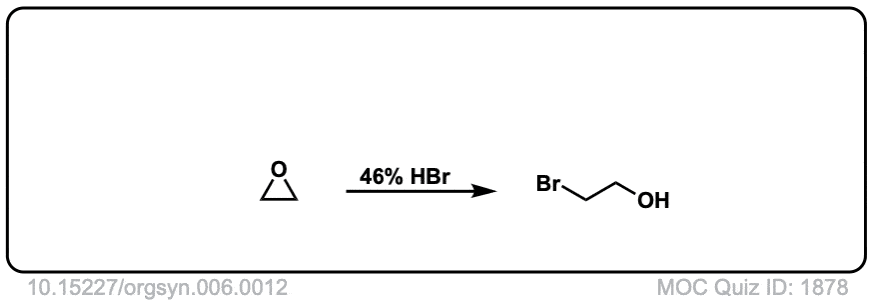
is this considered sn2?
It’s a bit like an SN1, except with inversion of stereochemistry. It’s very similar to the second step in bromination or chlorination of alkenes, where the nucleophile attacks the charged 3-membered ring intermediate.
Sir does the methyl substituent have any stereochemistry at the end?
yes, it is “up” in the example shown in the mechanism. The fact that OH is drawn as a “dash” implies that the methyl group is a “wedge” (up) although I was lazy and did not draw it in as such.
The reaction proceeds with inversion of configuration.
So helpful thank you!!!!