Formation of Acetals from Aldehydes and Ketones
Description: Ketones (or aldehydes) can be converted into acetals when treated with alcohols and acid. The byproduct is water.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
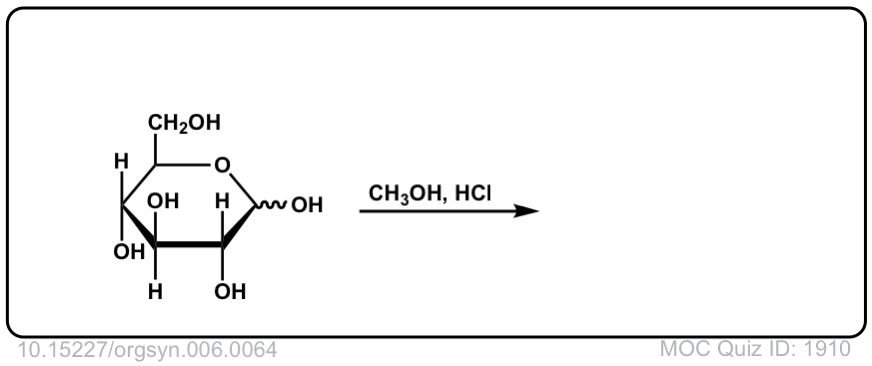
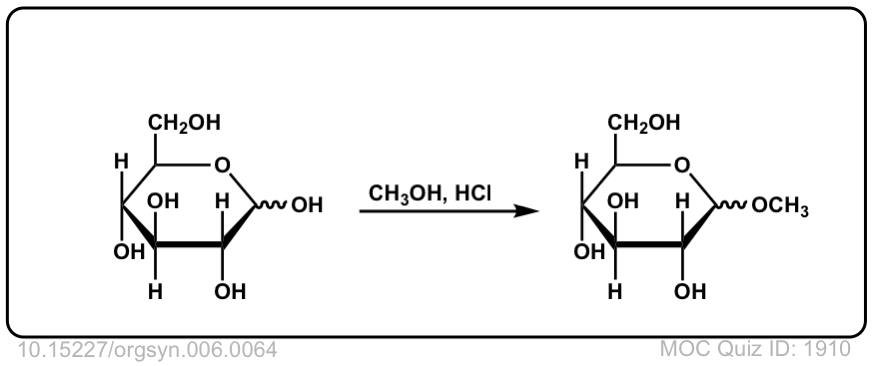
Org. Synth. 1926, 6, 64
DOI Link: 10.15227/orgsyn.006.0064
 Click to Flip
Click to Flip
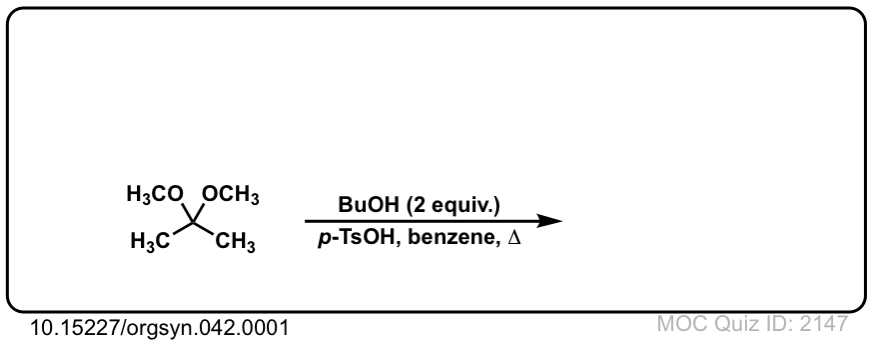
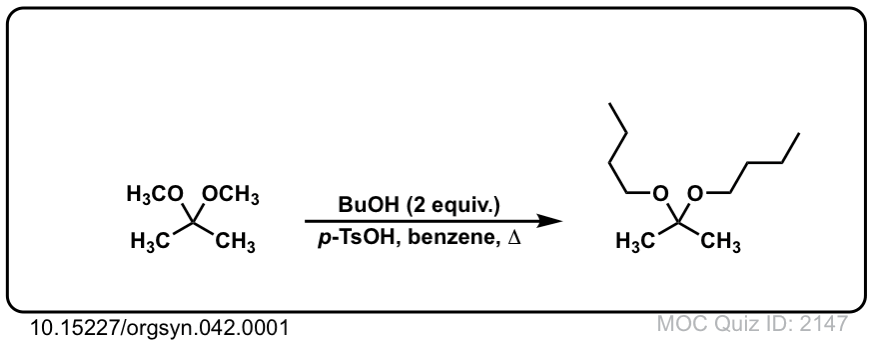
Org. Synth. 1962, 42, 1
DOI Link: 10.15227/orgsyn.042.0001
 Click to Flip
Click to Flip
Hi James!
Wow, sincere thanks for the video.
Just to clarify… You add a base to the reaction only when you need to deprotonate a H, like you did in Step 5. Otherwise, only the nucleophile will participate in addition reactions? Since protonating a ketone will make it more electrophilic, the strength of the nucleophile will not matter?
Thanks for this video!
You’re very welcome, thanks Brenda.