Oxidation of aldehydes to carboxylic acids using Cr(VI)
Description: Chromium trioxide (CrO3) and water will oxidize aldehydes to carboxylic acids.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
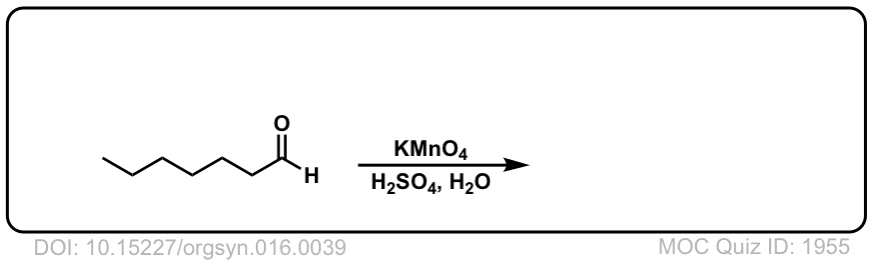
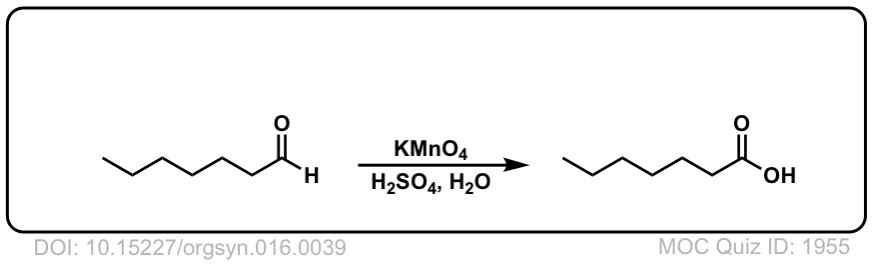
Org. Synth. 1936, 16, 39
DOI Link: 10.15227/orgsyn.016.0039
 Click to Flip
Click to Flip
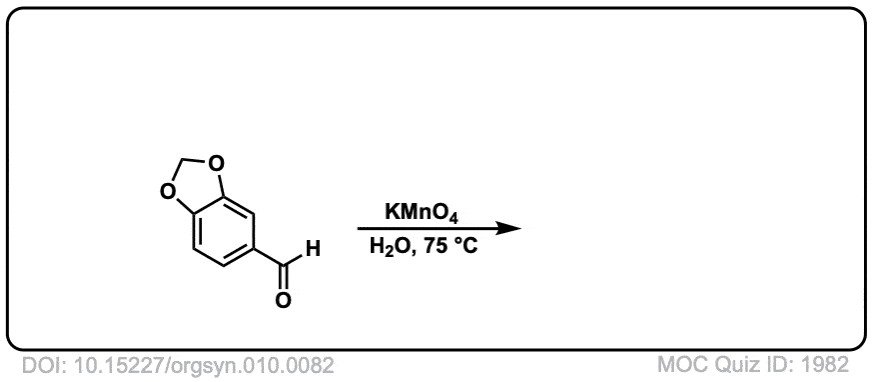
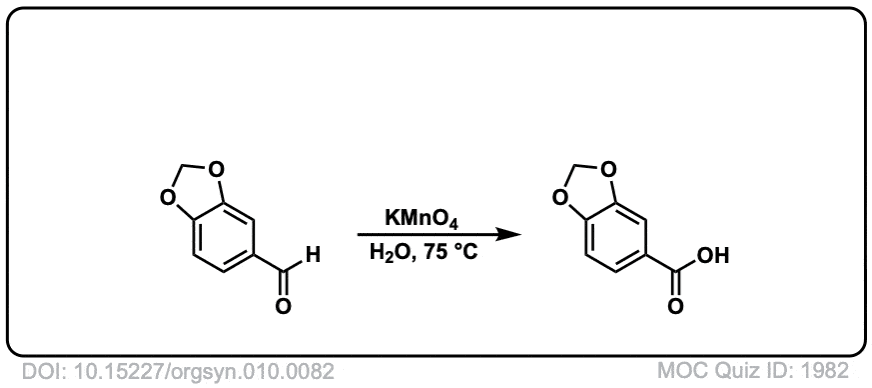
Org. Synth. 1930, 10, 82
DOI Link: 10.15227/orgsyn.010.0082
 Click to Flip
Click to Flip
Can a ketone be turned into a carboxylic acid?
Only indirectly, such as 1) through the haloform reaction (works only for methyl ketones) or 2) oxidation with a peroxyacid such as m-CPBA to give an ester, which can then be hydrolyzed to the carboxylic acid.
so i understand you can use bases for the last step to deprotonate the compound in the last step, but why do you have h2so4 in one of the examples? this would protonate right?
The purpose of H2SO4 is to protonate potassium dichromate, K2Cr2O7 to give chromic acid H2CrO4 which is the active oxidizing agent. Usually you’ll just see H+ but I wanted to be more specific. It’s not related to the last step. Water is a good enough base to do the elimination to give the carbonyl.
Thanks for all of the reactions! Your website is extremely helpful. Shouldn’t the reagent in example 3 say: K2Cr2O7?
Thanks again!
Yes – fixed. Thank you!