Aldol addition reaction of aldehydes and ketones
Description: The Aldol reaction is the addition of an enolate ion with an aldehyde or ketone.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Examples:
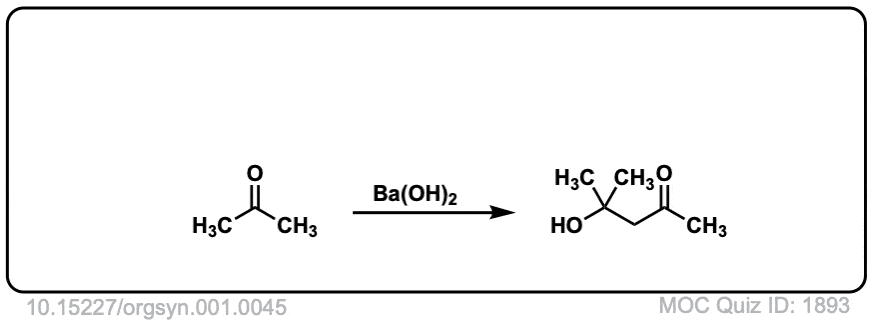
Org. Synth. 1921, 1, 45
DOI Link: 10.15227/orgsyn.001.0045
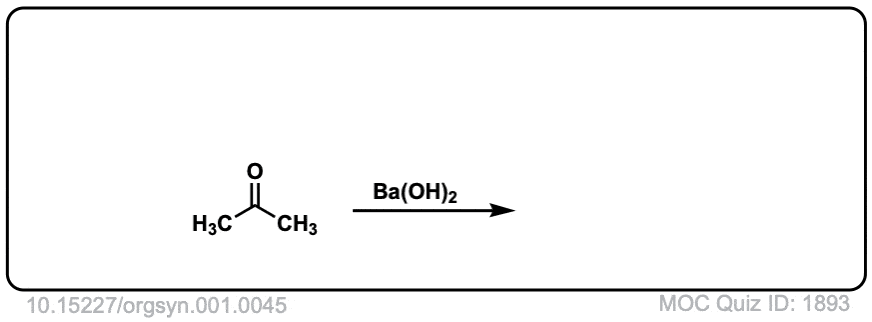 Click to Flip
Click to Flip
What would a case of two ketones look like?
If 2 equivalents of aldehyde were reacted with one equivalent of ketone would the third step be changed so that the 1,2 addition product would undergo another addition by the aldehyde?
Not 100% sure I’m visualizing what you’re asking, but let’s take the example of the reaction of the ketone 2-propanone with benzaldehyde (2 equivalents). The first aldol reaction would be deprotonation of propanone to give the enolate followed by addition to benzaldehyde. Then, you could certainly form an enolate on the other side and do an aldol addition to a second equivalent of benzaldehyde. This is a known reaction and after dehydration the final product is dibenzylidene acetone https://en.wikipedia.org/wiki/Dibenzylideneacetone
Hey James,
Broadly speaking, why does the base (any base, LDA, NaOH, etc.) deprotonate the hydrogen on the alpha carbon, as opposed to deprotonating the aldehyde’s lone Hydrogen? I know that enol/enolate stuff is called “Alpha-Carbon” chemistry for a reason, but I’m curious as to what the reason is (sterics, stability– they should be equally resonance-stabilized, no?)
Thanks for an awesome website!
Hi, is there possibility to use Na2CO3 like a base? What would be different? Thx, P.
Sure, one could use Na2CO3 as a base – it’s probably reacting with water present in solution to give trace amounts of HO- , which is doing the deprotonation. Na2CO3 isn’t the greatest base in the world however.
Hi,
I am slightly confused about the first two examples as it seems that the enolate formed from the least substituted carbonyl compound without the use of LDA?
Many thanks for an excellent website.
Aha! Look more closely… what enolates are possible here?