Alkylation of enolates
Description: Enolates of carbonyl compounds will react with alkyl halides in SN2 reactions to give alkylation products.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-Life Example:
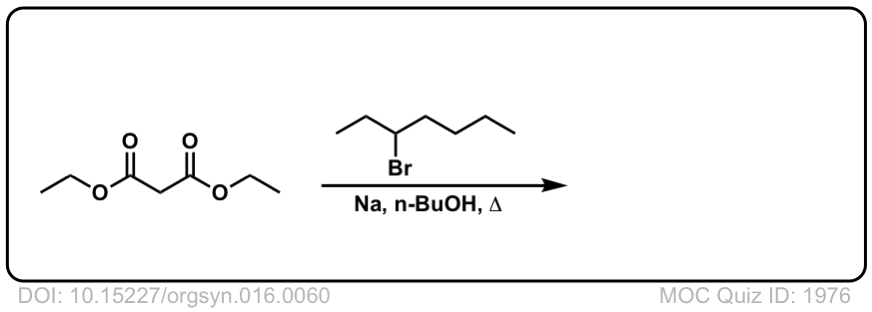
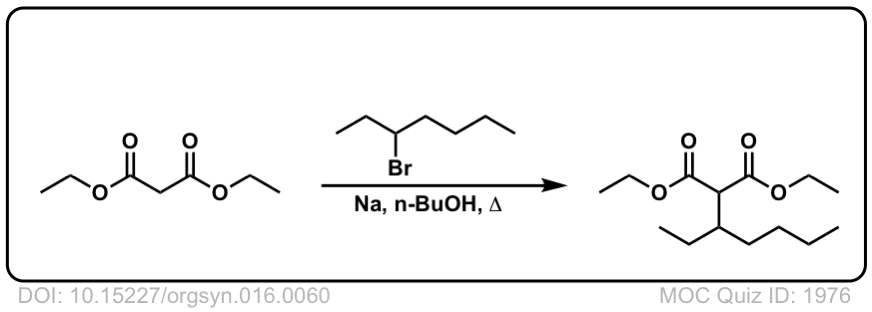
Org. Synth. 1936, 16, 60
DOI Link: 10.15227/orgsyn.016.0060
 Click to Flip
Click to Flip
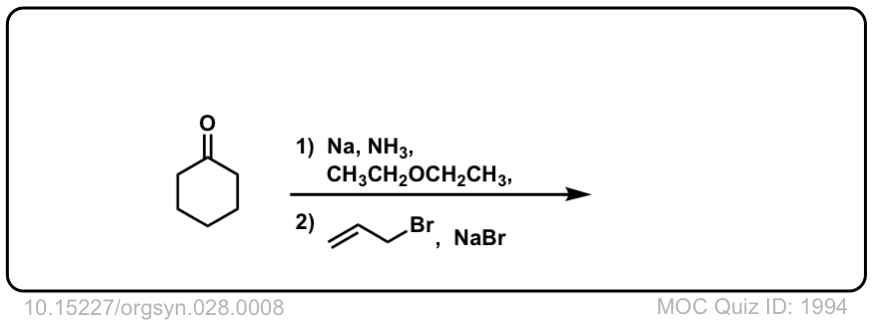
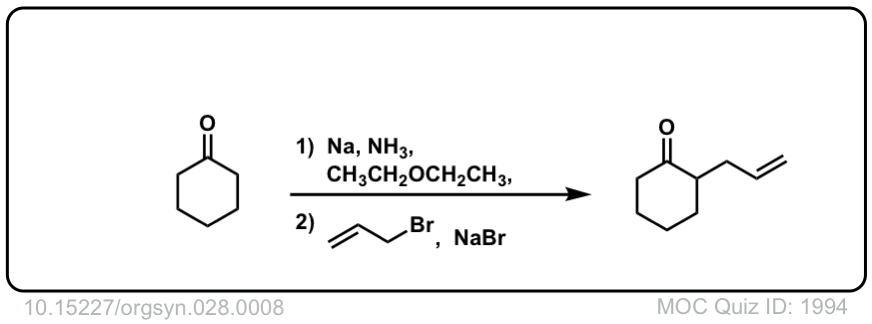
Org. Synth. 1948, 28, 8
DOI Link: 10.15227/orgsyn.028.0008
 Click to Flip
Click to Flip
Org. Synth. 1948, 28, 8
DOI Link: 10.15227/orgsyn.021.0099
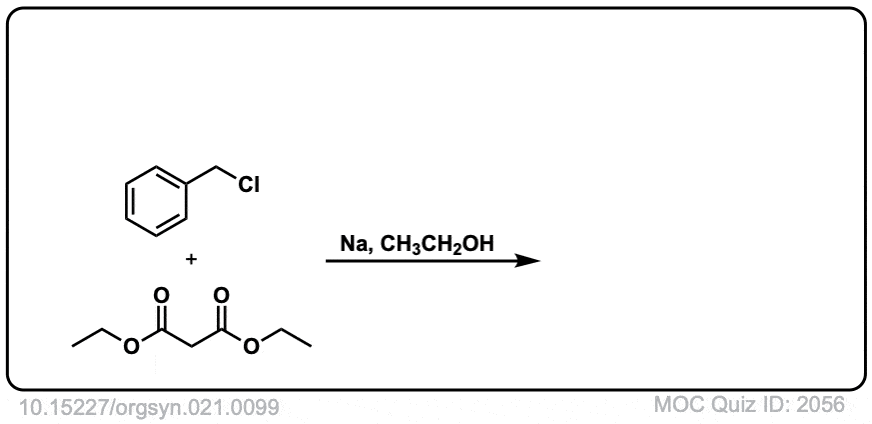 Click to Flip
Click to Flip
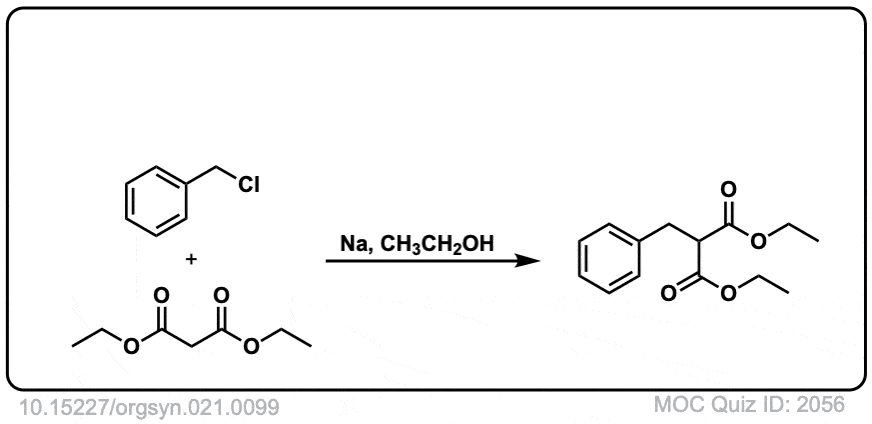
There’s just a little different: the last quiz shows ID:1781 on the front but ID:1781 on the back.
Thank you for showing so many examples.
That’s really helpful.
Thank you for spotting the typo. Don’t stop!
Why in the fourth reaction was formed the least substituted enolate? More substituted enolate (and pi bond) wouldn’t be more stable?
Great site!
Great question. Lithium diisopropyl amide (LDA) is a bulky base and forms the least substituted enolate upon deprotonation. https://www.masterorganicchemistry.com/2011/08/05/reagent-friday-lithium-di-isopropyl-amide-lda/