Conversion of acid chlorides to esters through addition of an alcohol
Description: Acid chlorides are converted to esters when treated with alcohols.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real-World Examples:
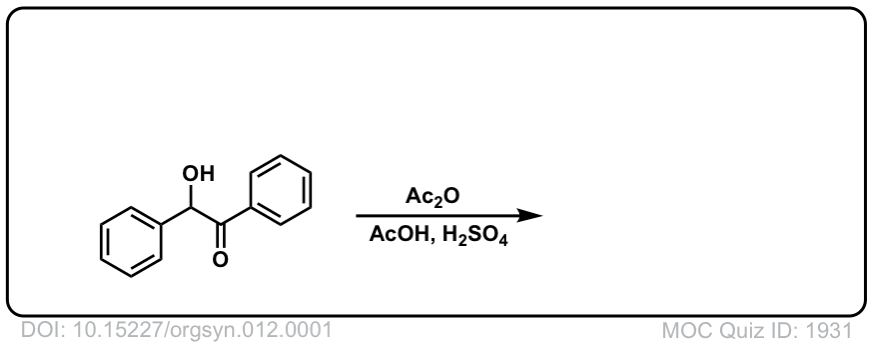
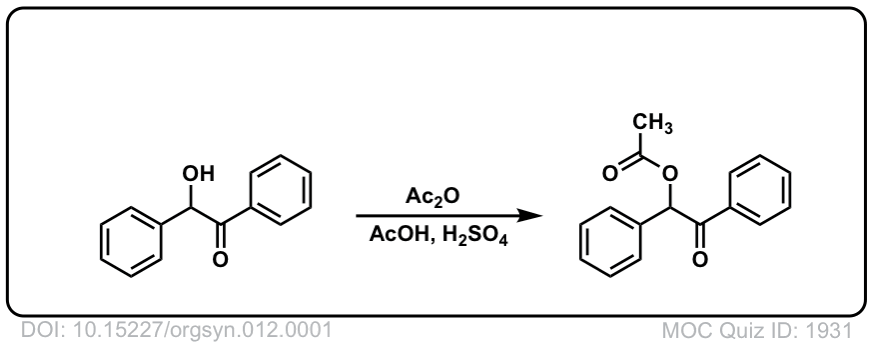
Org. Synth. 1932, 12, 1
DOI Link: 10.15227/orgsyn.012.0001
 Click to Flip
Click to Flip
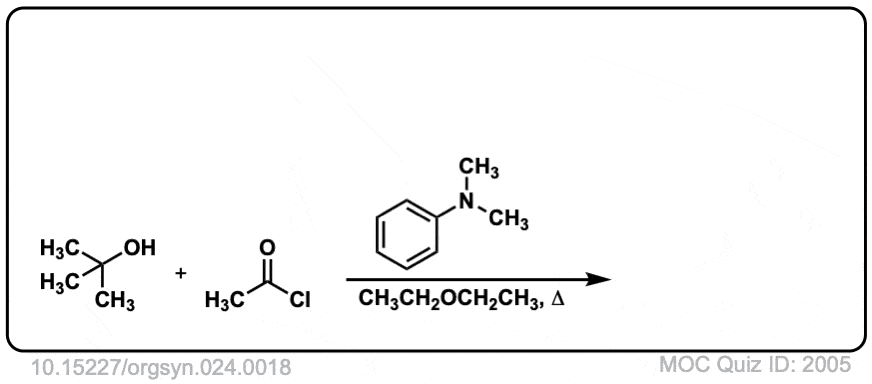
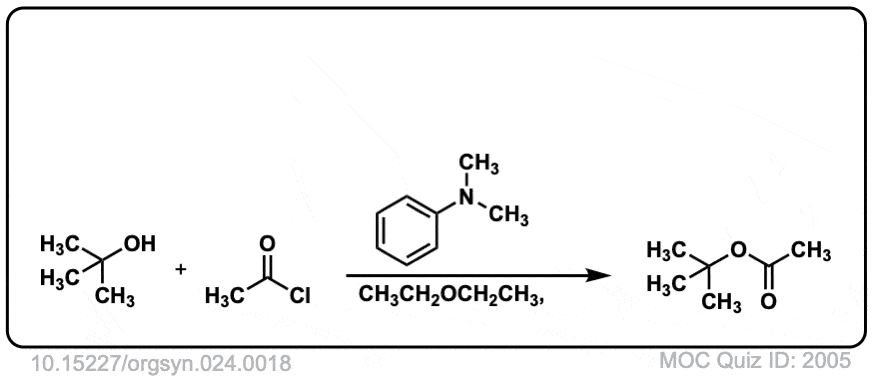
Org. Synth. 1944, 24, 18
DOI Link: 10.15227/orgsyn.024.0018
 Click to Flip
Click to Flip
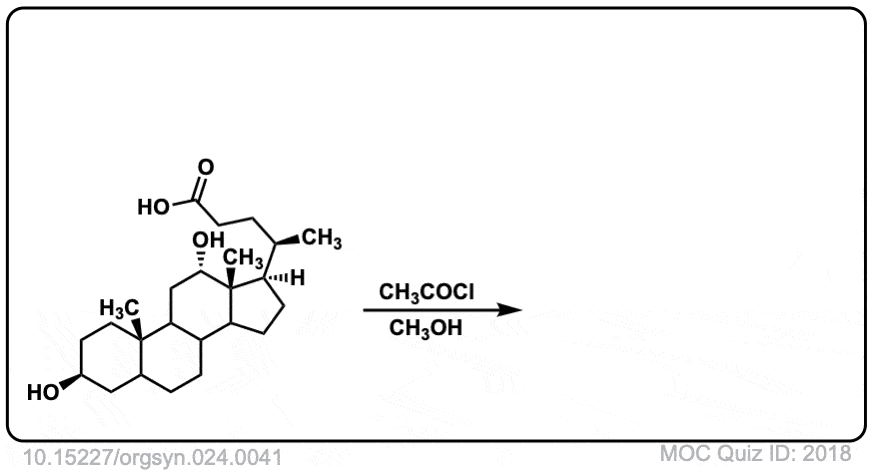
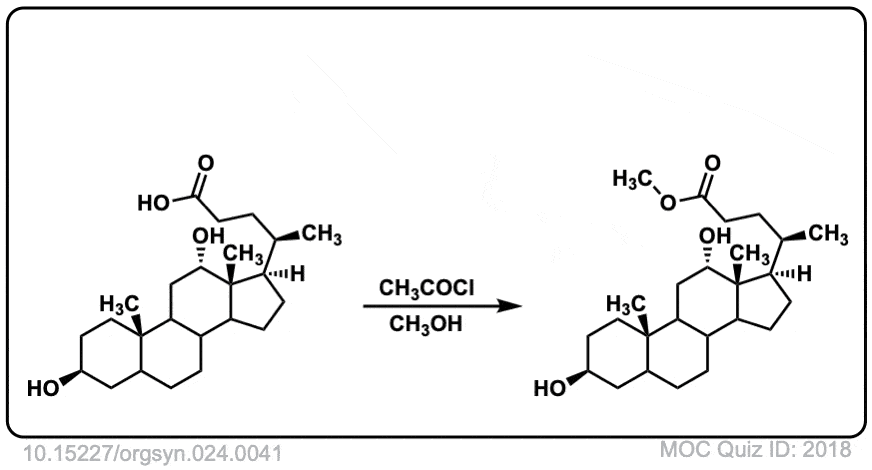
Org. Synth. 1944, 24, 41
DOI Link: 10.15227/orgsyn.024.0041
 Click to Flip
Click to Flip
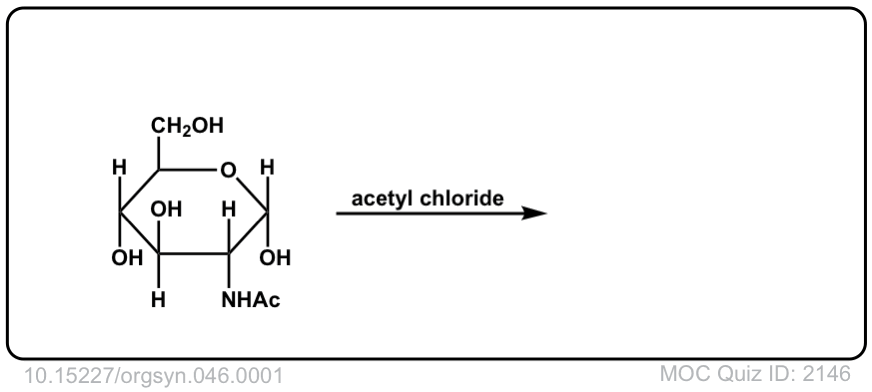
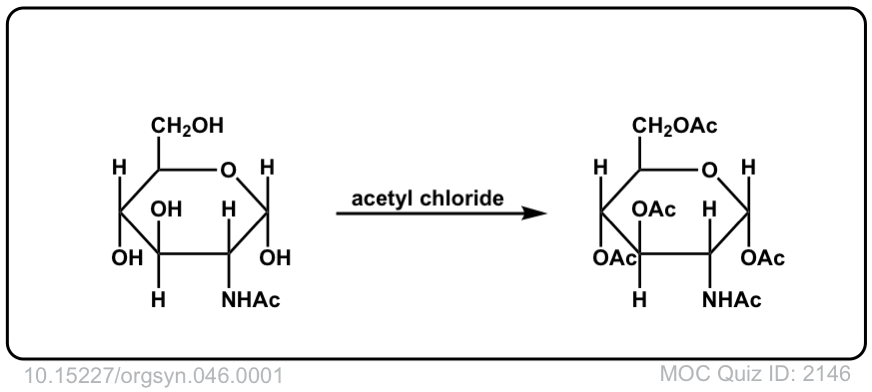
Org. Synth. 1966, 46, 1
DOI Link: 10.15227/orgsyn.046.0001
 Click to Flip
Click to Flip
Org. Synth. 1971, 51, 96
DOI Link: 10.15227/orgsyn.051.0096
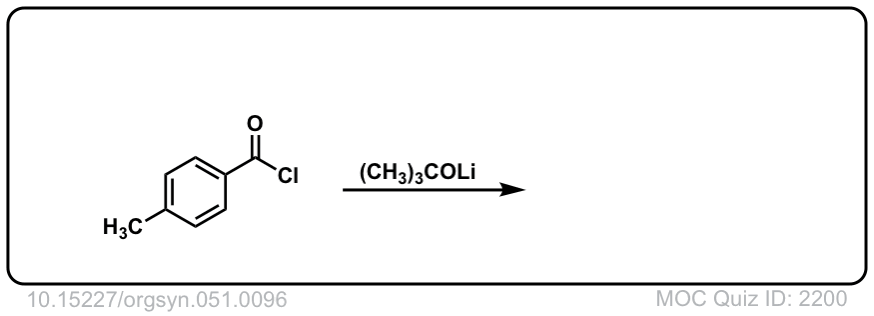 Click to Flip
Click to Flip
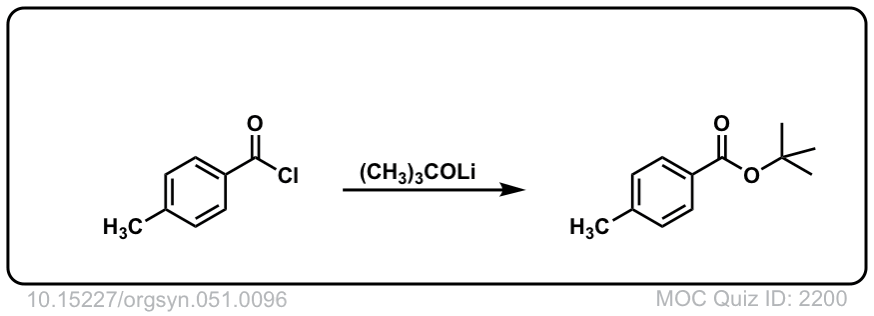
Our textbook has this reaction shown only with acid chlorides, as well. Does the addition-elimination happen with other acid halides or just acid chloride?
do you have a mechanism explain the reaction between alcoholic alkoxide with acyl halide or anhydride to form ester ??
I’m interested in to make a reaction between alcohol and maleic anhydride , but i don’t know what is the mechanism of the reaction will occur ..
may you help me please ??
Thanks ,
Ameen
It’s the same as this, but there is no proton transfer step. Addition and then elimination.