The Mannich Reaction
Description: The Mannich reaction is similar to the Aldol reaction, except that instead of an enol (or enolate) adding to an aldehyde or ketone, it adds to an imine (or iminium).
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
Real=Life Examples:
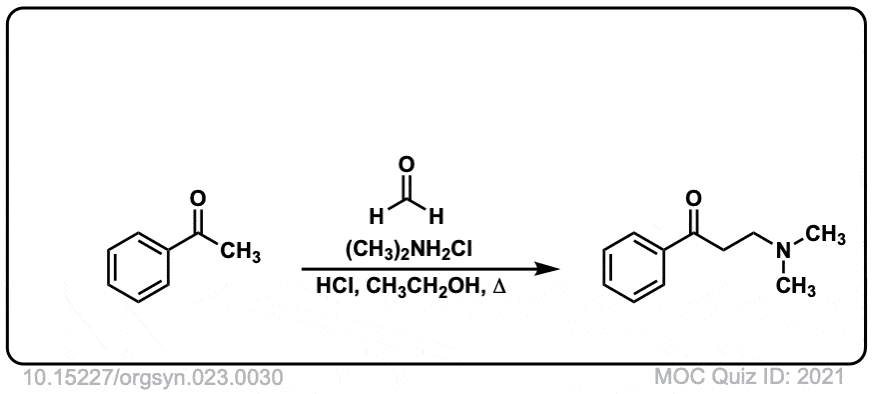
Org. Synth. 1943, 23, 30
DOI Link: 10.15227/orgsyn.023.0030
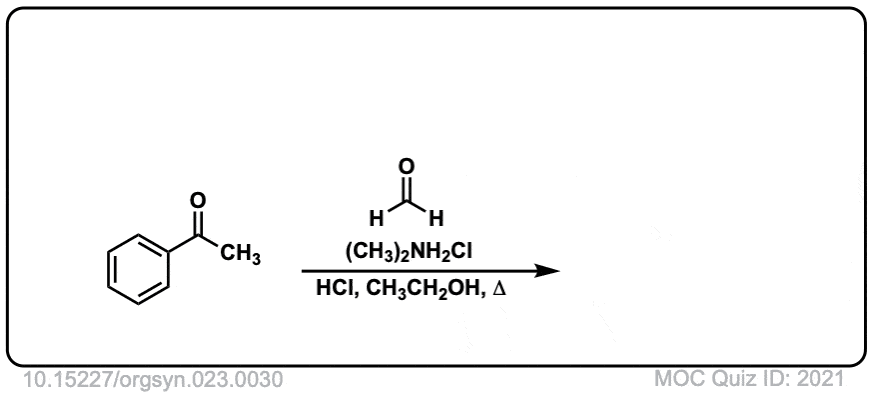 Click to Flip
Click to Flip
Hi, this reaction is under acidic conditions and will go through the enol. The most favorable enol is in between the two carbonyl groups as this will have an internal hydrogen bond between the OH and the carbonyl oxygen.
If you wanted to get the Mannich to happen on the less substituted carbon the way to do it would be to add LDA and then the iminium.
I wondered that about quiz#1840, how should I choose the attacked site by iminium?
I preferred the methyl group beside the carbonyl(not the ester one) because the steric hindrance of the product would be less than the one which attacked position is between the two carbonyl group
Thanks for your reading!