Allylic bromination of alkanes using NBS
Description: When treated with N-bromosuccinimide (NBS) and light (hν) alkyl groups adjacent to alkenes will be converted into alkyl bromides.
The rest of this page is available to MOC Members only.
To get access to this page, plus over 1500 quizzes, the Reaction Encyclopedia, Org 1 / Org 2 summary sheets, and flashcards, sign up here for only 30 cents/ day!
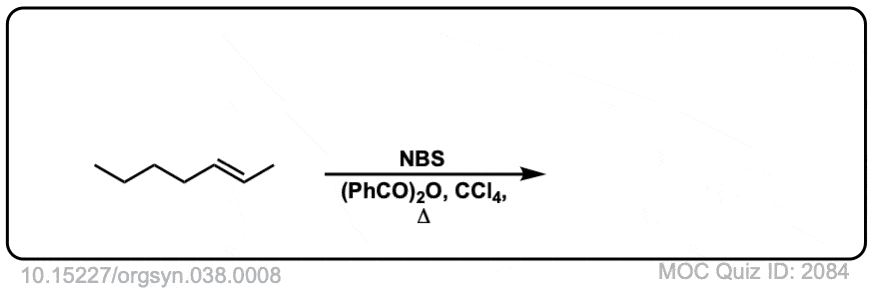
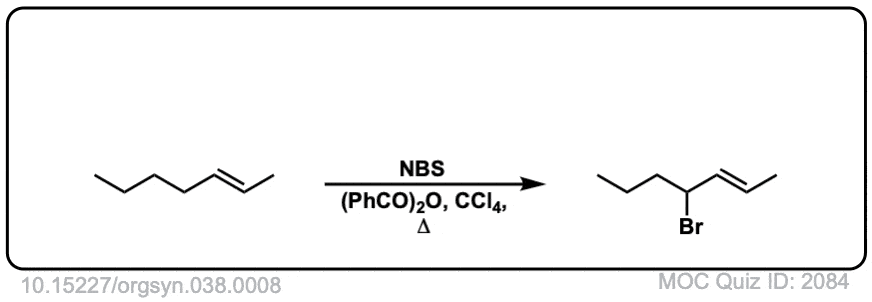
Real-World Example:
Org. Synth. 1958, 38, 8
DOI Link: 10.15227/orgsyn.038.0008
 Click to Flip
Click to Flip
Hey, how can the fourth reaction in your example take place if neither heat nor light is present?
Good point – should probably put “heat” in there. Thank you!
Fixed. thanks again!
When substituting NBS for Br2, only 1 eq is required?
Hi James,
I’m confused by the presence of Br2. How exactly is it formed? My textbook says that it is an ionic reaction between HBr and NBS but I can’t find any sources that show this mechanism. Thank you!
Great question! the generation of br2 from nbs and hbr would look something like this: https://www.masterorganicchemistry.com/wp-content/uploads/2019/12/Br2-from-nbs-and-hbr-1.gif
Hi James,
Will this reaction brominate at the benzylic position as well?
More specifically, can NBS brominate toluene?
Yes, benzylic bromination is a very well known reaction.
does NBS brominate benzylic position?
Yes it does.
In your example using NBS in combination with peroxide and heat what, if anything, would happen differently if NBS was used in combination with only peroxide?
In the absence of heat, the O-O bond of the peroxide won’t be broken, and the free-radical reaction won’t start. Heat is required for initiation.
Hey you have in all radical reaction the same mistake D comes by bonds broken and E by bonds formed ;)
Fixed. Thank you.
I couldn’t understand very well if 1 mole of NBS will give me, in theory, 1 mole of brominated product. Besides, will I get only 0.5 moles of brominated product when I use pure bromine (due to the formation of HBr)? Any help would be appreciated.
Hi Felipe, Low yield is the main issue in this reaction. I think if we use little HBr with NBS so can improve the yield. My opinion is that adding HBr can prevent NBS to Make HBr initially.
Greetings! I have found your Oxidation Ladder, part of your Org 2 study sheets to be invaluable. I have “added” allylic bromination to your oxidation ladder. You should definitely add it into a future version.
Hi Frederick – thanks for writing and I’m very glad you found that sheet useful – it’s one of my favorites. I agree that allylic bromination is an oxidation but I wasn’t sure where to put it in the oxidation ladder that would make sense in the context of other reactions. For instance one could do an elimination to make a diene, but there are no dienes on the sheet. So I left it off in the name of simplicity. Where did you add it, if you don’t mind me asking?
In the subject of this post you have written alkanes and these are, I think alkenes.
I chose “alkanes” because it’s an alkyl (sp3) carbon which is being brominated but I should probably just remove the “alkanes” part and keep it as “allylic bromination”. : – )
Hey from where exactly are you getting the HBr
The dirty secret with this reaction is that it works best with slightly impure NBS, which contains HBr.
Hi James, what do you mean by impure NBS. Do you mean that for best reaction we should add little HBr to the reaction mixture with pure NBS?
Pure NBS is gleaming white crystals. After sitting on the shelf for awhile, it often gains a yellow color due to trace Br2 and HBr. That grade of NBS is perfect for allylic bromination because it already contains trace Br2.
Hey I think you are wrong because in the third and fourth reaction from top you forgot allylic rearrangement
Good point! This should be updated to reflect the potential for allylic rearrangement.
Has this been corrected?
Yes, it’s been fixed!
what is the reagent used in allylic bromination of CH3CH2CH=CH2
NBS, just like in this example.
In the subject of this post you have written alkanes and these are, I think alkenes.
Because it’s a bromination of the allylic carbon I think it still qualifies as an alkane-centric reaction, as the double bond isn’t involved in the mechanism beyond resonance stabilization of the carbon radical.